Abstract
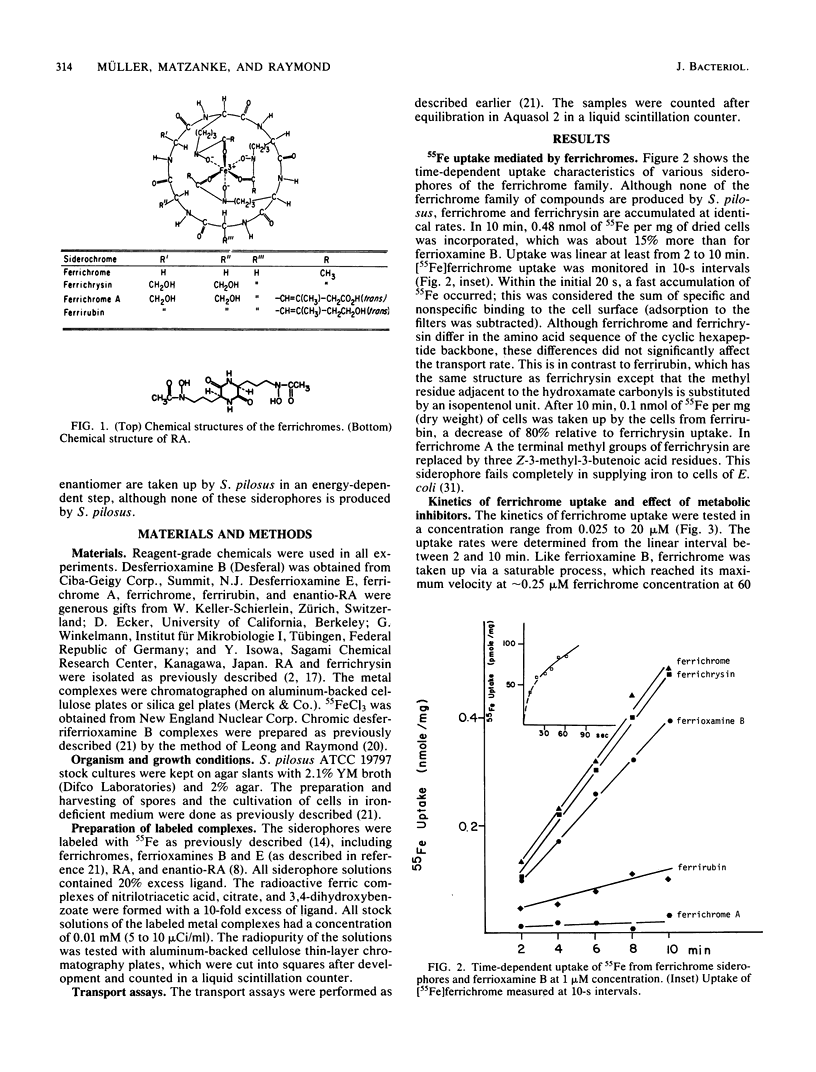
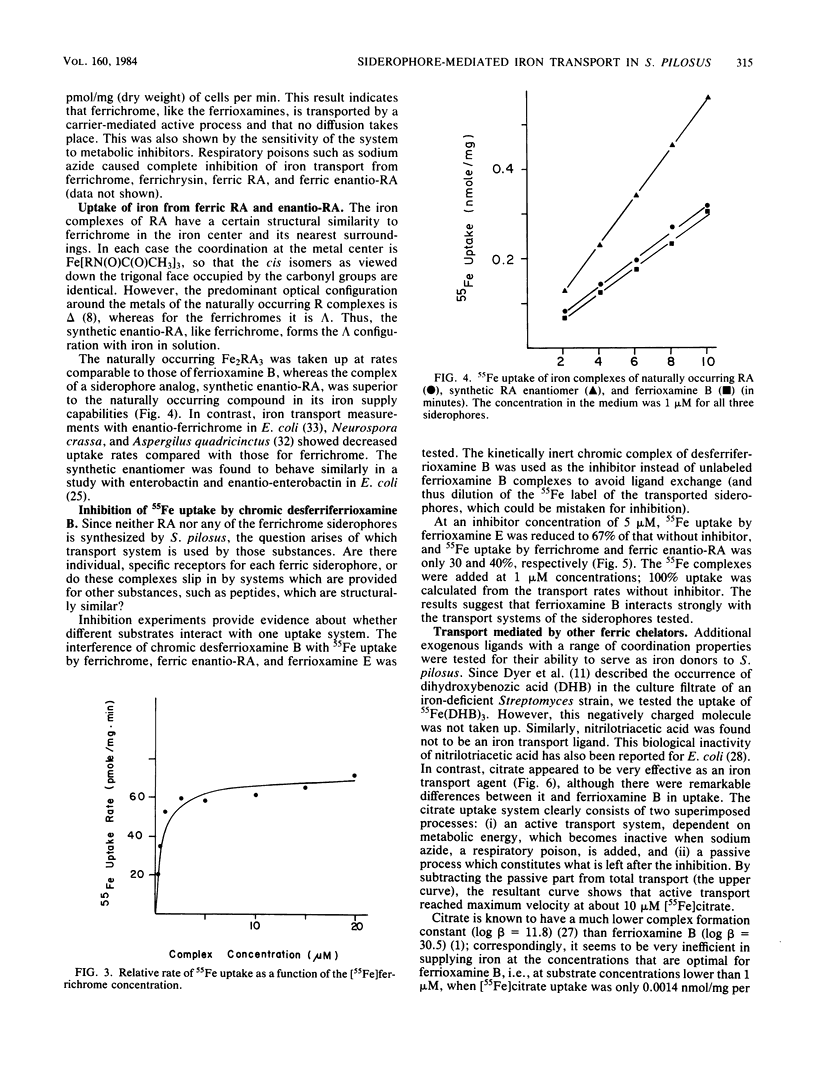
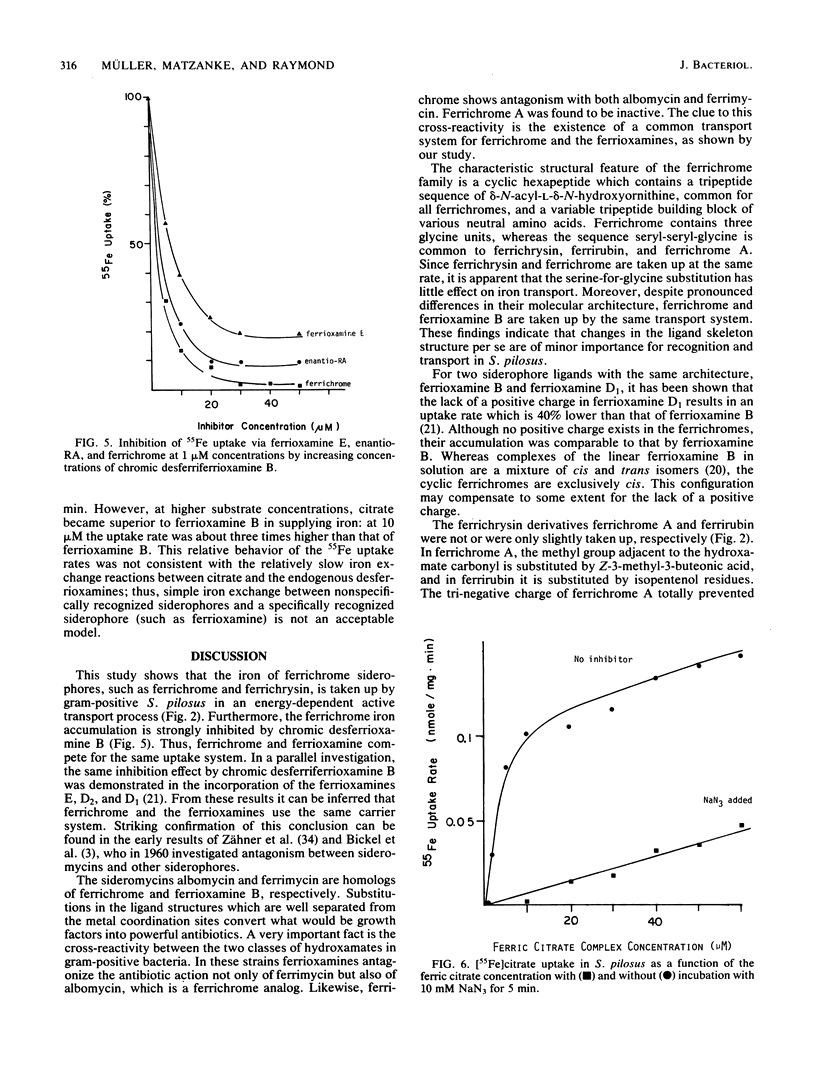
Streptomyces pilosus is one of several microbes which produce ferrioxamine siderophores. In the accompanying paper (G. Müller and K. Raymond, J. Bacteriol. 160:304-312), the mechanism of iron uptake mediated by the endogenous ferrioxamines B, D1, D2, and E was examined. Here we report iron transport behavior in S. pilosus as mediated by the exogenous siderophores ferrichrome, ferrichrysin, rhodotorulic acid (RA), and synthetic enantio-RA. In each case iron acquisition depended on metabolic energy and had uptake rates comparable to that of [55Fe]ferrioxamine B. However, the synthetic ferric enantio-RA (which has the same preferred chirality at the metal center as ferrichrome) was twice as effective in supplying iron as was the natural ferric RA complex, suggesting that stereospecific recognition at the metal center is involved in the transport process. Iron uptake mediated by ferrichrome and ferric enantio-RA was strongly inhibited by kinetically inert chromic complexes of desferrioxamine B. These inhibition experiments indicate that iron from these exogenous siderophores is transported by the same uptake system as ferrioxamine B. Since the ligands have no structural similarity to ferrioxamine B except for the presence of three hydoxamate groups, we conclude that only the hydroxamate iron center and its direct surroundings are important for recognition and uptake. This hypothesis is supported by the fact that ferrichrome A and ferrirubin, which are both substituted at the hydroxamate carbonyl groups, were not (or were poorly) effective in supplying iron to S. pilosus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin C. L., Neilands J. B. Rhodotorulic acid, a diketopiperazine dihydroxamic acid with growth-factor activity. I. Isolation and characterization. Biochemistry. 1968 Oct;7(10):3734–3739. doi: 10.1021/bi00850a054. [DOI] [PubMed] [Google Scholar]
- BICKEL H., GAEUMANN E., KELLER-SCHIERLEIN W., PRELOG V., VISCHER E., WETTSTEIN A., ZAEHNER H. [On iron-containing growth factors, sideramines, and their antagonists, the iron-containing antibiotics, sideromycins]. Experientia. 1960 Apr 15;16:129–133. doi: 10.1007/BF02157712. [DOI] [PubMed] [Google Scholar]
- Braun V., Burkhardt R., Schneider R., Zimmermann L. Chromosomal genes for ColV plasmid-determined iron(III)-aerobactin transport in Escherichia coli. J Bacteriol. 1982 Aug;151(2):553–559. doi: 10.1128/jb.151.2.553-559.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrano C. J., Raymond K. N. Coordination chemistry of microbial iron transport compounds: rhodotorulic acid and iron uptake in Rhodotorula pilimanae. J Bacteriol. 1978 Oct;136(1):69–74. doi: 10.1128/jb.136.1.69-74.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Naegeli H. U., Braun V. Iron supply of Escherichia coli with polymer-bound ferricrocin. Eur J Biochem. 1979 Aug 15;99(1):39–47. doi: 10.1111/j.1432-1033.1979.tb13228.x. [DOI] [PubMed] [Google Scholar]
- Coulton J. W. The ferrichrome-iron receptor of Escherichia coli K-12. Antigenicity of the fhuA protein. Biochim Biophys Acta. 1982 Jul 16;717(1):154–162. doi: 10.1016/0304-4165(82)90393-2. [DOI] [PubMed] [Google Scholar]
- Ecker D. J., Emery T. Iron uptake from ferrichrome A and iron citrate in Ustilago sphaerogena. J Bacteriol. 1983 Aug;155(2):616–622. doi: 10.1128/jb.155.2.616-622.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker D. J., Lancaster J. R., Jr, Emery T. Siderophore iron transport followed by electron paramagnetic resonance spectroscopy. J Biol Chem. 1982 Aug 10;257(15):8623–8626. [PubMed] [Google Scholar]
- Emery T. Role of ferrichrome as a ferric ionophore in Ustilago sphaerogena. Biochemistry. 1971 Apr 13;10(8):1483–1488. doi: 10.1021/bi00784a033. [DOI] [PubMed] [Google Scholar]
- Leong J., Raymond K. N. Coordination isomers of biological iron transport compounds. II. The optical isomers of chromic desferriferrichrome and desferriferrichrysin. J Am Chem Soc. 1974 Oct 16;96(21):6628–6630. doi: 10.1021/ja00828a014. [DOI] [PubMed] [Google Scholar]
- Müller G., Raymond K. N. Specificity and mechanism of ferrioxamine-mediated iron transport in Streptomyces pilosus. J Bacteriol. 1984 Oct;160(1):304–312. doi: 10.1128/jb.160.1.304-312.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B., Erickson T. J., Rastetter W. H. Stereospecificity of the ferric enterobactin receptor of Escherichia coli K-12. J Biol Chem. 1981 Apr 25;256(8):3831–3832. [PubMed] [Google Scholar]
- Neilands J. B. Iron absorption and transport in microorganisms. Annu Rev Nutr. 1981;1:27–46. doi: 10.1146/annurev.nu.01.070181.000331. [DOI] [PubMed] [Google Scholar]
- Norrestam R., Stensland B., Brändén C. I. On the conformation of cyclic iron-containing hexapeptides: the crystal and molecular structure of ferrichrysin. J Mol Biol. 1975 Dec 15;99(3):501–506. doi: 10.1016/s0022-2836(75)80141-0. [DOI] [PubMed] [Google Scholar]
- Wayne R., Frick K., Neilands J. B. Siderophore protection against colicins M, B, V, and Ia in Escherichia coli. J Bacteriol. 1976 Apr;126(1):7–12. doi: 10.1128/jb.126.1.7-12.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAEHNER H., HUETTER R., BACHMANN E. [Metabolites of Actinomycetes. Part 23. On a study of the effect of sideromycin]. Arch Mikrobiol. 1960;36:325–349. [PubMed] [Google Scholar]
- Zalkin A., Forrester J. D., Templeton D. H. Ferrichrome-A tetrahydrate. Determination of crystal and molecular structure. J Am Chem Soc. 1966 Apr 20;88(8):1810–1814. doi: 10.1021/ja00960a040. [DOI] [PubMed] [Google Scholar]


