Abstract
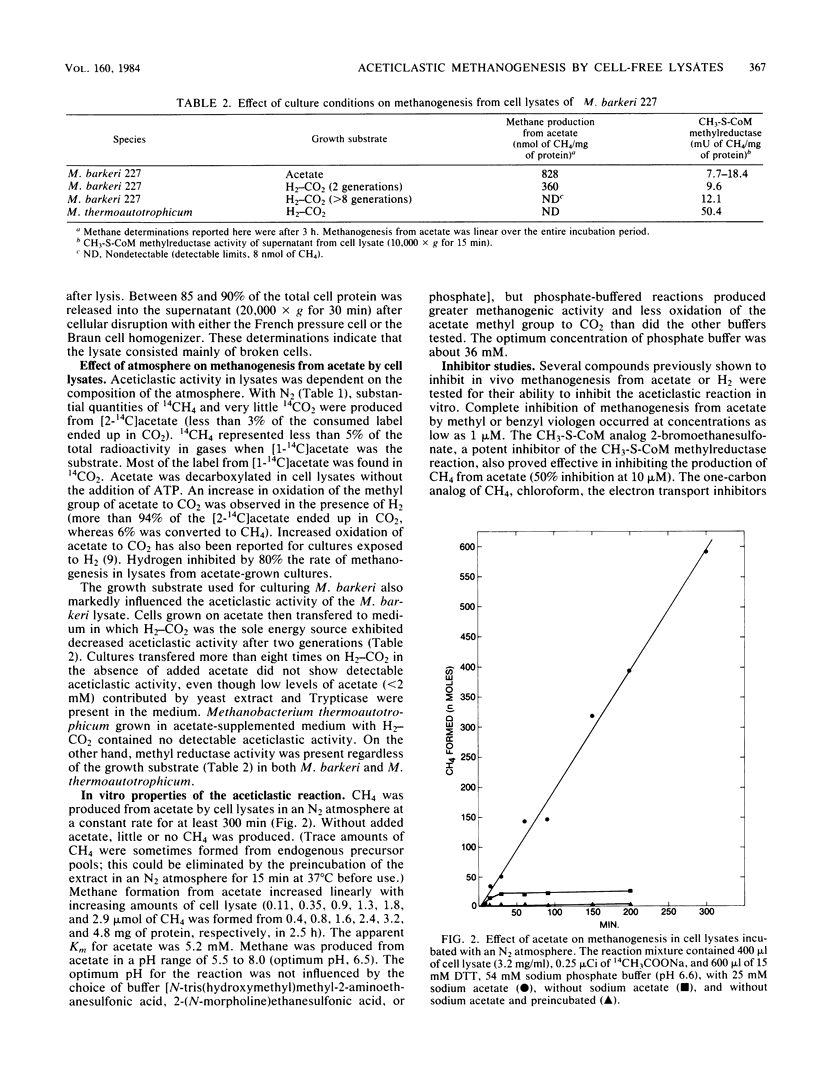
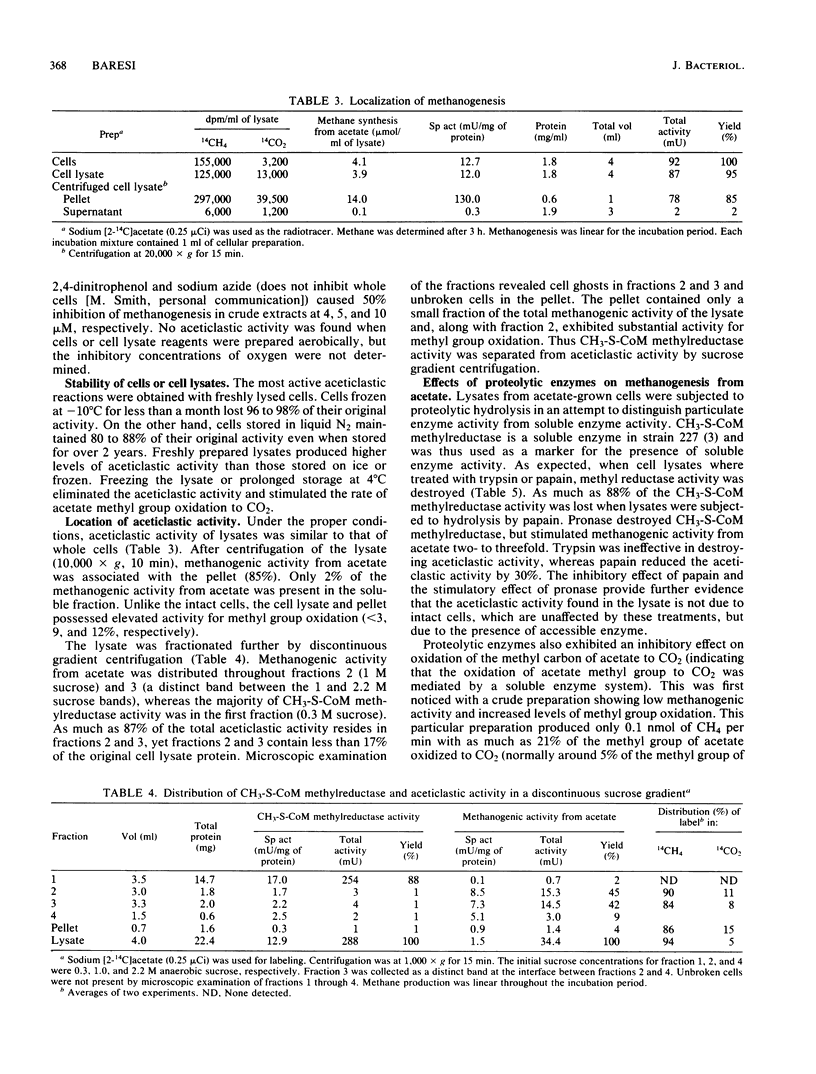
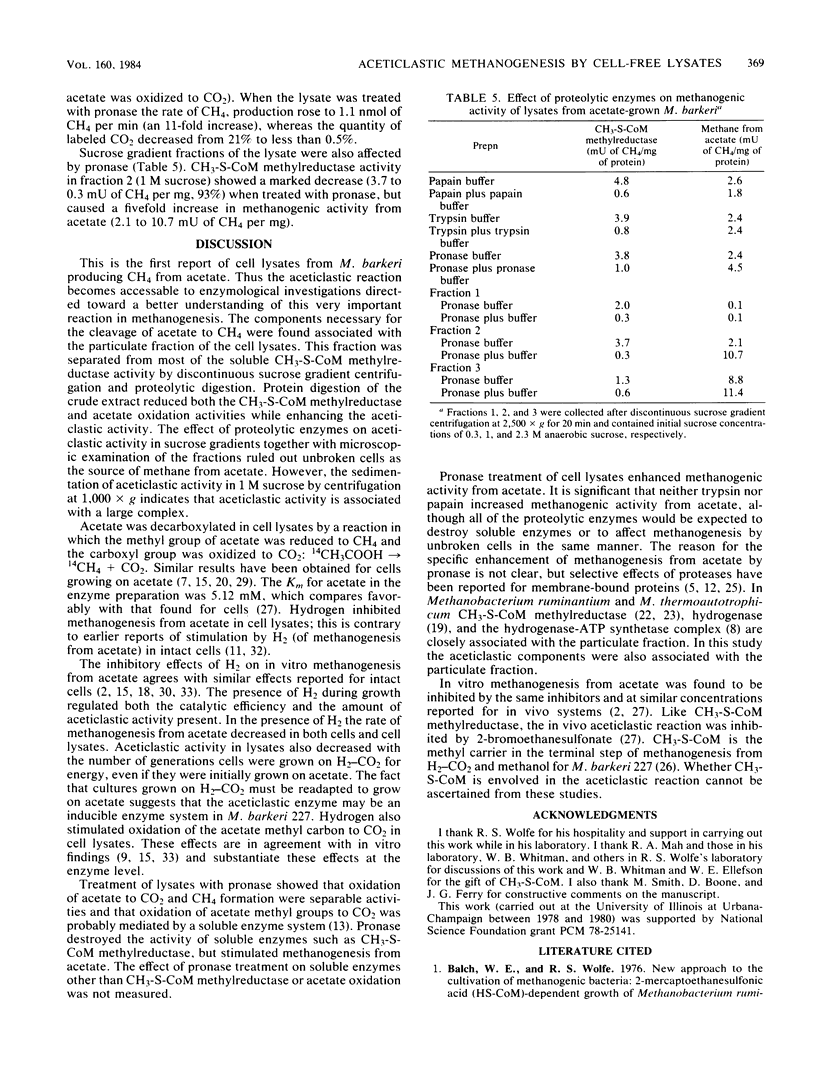
Cell lysates of acetate-grown Methanosarcina barkeri 227 were found to cleave acetate to CH4 and CO2. The aceticlastic reaction was identified by using radioactive methyl-labeled acetate. Cell lysates decarboxylated acetate in a nitrogen atmosphere, conserving the methyl group in methane. The rate of methanogenesis from acetate in the cell lysates was comparable to that observed with whole cells. Aceticlastic activity was found in the particulate fraction seperate from methylcoenzyme M methylreductase activity, which occurs in the soluble fraction. Pronase treatment eliminated methylcoenzyme M methylreductase activity in lysates and stimulated aceticlastic activity, indicating the aceticlastic activity was not derived from unbroken cells, which are unaffected by proteolytic treatment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baresi L., Mah R. A., Ward D. M., Kaplan I. R. Methanogenesis from acetate: enrichment studies. Appl Environ Microbiol. 1978 Jul;36(1):186–197. doi: 10.1128/aem.36.1.186-197.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baresi L., Wolfe R. S. Levels of coenzyme F420, coenzyme M, hydrogenase, and methylcoenzyme M methylreductase in acetate-grown Methanosarcina. Appl Environ Microbiol. 1981 Feb;41(2):388–391. doi: 10.1128/aem.41.2.388-391.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker H. A. Studies on the Methane Fermentation: VI. The Influence of Carbon Dioxide Concentration on the Rate of Carbon Dioxide Reduction by Molecular Hydrogen. Proc Natl Acad Sci U S A. 1943 Jun;29(6):184–190. doi: 10.1073/pnas.29.6.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doddema H. J., van der Drift C., Vogels G. D., Veenhuis M. Chemiosmotic coupling in Methanobacterium thermoautotrophicum: hydrogen-dependent adenosine 5'-triphosphate synthesis by subcellular particles. J Bacteriol. 1979 Dec;140(3):1081–1089. doi: 10.1128/jb.140.3.1081-1089.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson T. J., Mah R. A. Effect of H(2)-CO(2) on Methanogenesis from Acetate or Methanol in Methanosarcina spp. Appl Environ Microbiol. 1983 Aug;46(2):348–355. doi: 10.1128/aem.46.2.348-355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. ATP activation and properties of the methyl coenzyme M reductase system in Methanobacterium thermoautotrophicum. J Bacteriol. 1978 Sep;135(3):851–857. doi: 10.1128/jb.135.3.851-857.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutten T. J., Bongaerts H. C., van der Drift C., Vogels G. D. Acetate, methanol and carbon dioxide as substrates for growth of Methanosarcina barkeri. Antonie Van Leeuwenhoek. 1980;46(6):601–610. doi: 10.1007/BF00394016. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L. Purification and characterization of (Na+ + K+)-ATPase. VI. Differential tryptic modification of catalytic functions of the purified enzyme in presence of NaCl and KCl. Biochim Biophys Acta. 1977 Apr 1;466(1):97–108. doi: 10.1016/0005-2736(77)90211-5. [DOI] [PubMed] [Google Scholar]
- Krzycki J. A., Wolkin R. H., Zeikus J. G. Comparison of unitrophic and mixotrophic substrate metabolism by acetate-adapted strain of Methanosarcina barkeri. J Bacteriol. 1982 Jan;149(1):247–254. doi: 10.1128/jb.149.1.247-254.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mah R. A., Smith M. R., Baresi L. Studies on an acetate-fermenting strain of Methanosarcina. Appl Environ Microbiol. 1978 Jun;35(6):1174–1184. doi: 10.1128/aem.35.6.1174-1184.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah R. A., Ward D. M., Baresi L., Glass T. L. Biogenesis of methane. Annu Rev Microbiol. 1977;31:309–341. doi: 10.1146/annurev.mi.31.100177.001521. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McInerney M. J., Bryant M. P. Anaerobic Degradation of Lactate by Syntrophic Associations of Methanosarcina barkeri and Desulfovibrio Species and Effect of H(2) on Acetate Degradation. Appl Environ Microbiol. 1981 Feb;41(2):346–354. doi: 10.1128/aem.41.2.346-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar R. C., Sprott G. D. Solubilization and properties of a particulate hydrogenase from Methanobacterium strain G2R. J Bacteriol. 1979 Jul;139(1):231–238. doi: 10.1128/jb.139.1.231-238.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINE M. J., VISHNIAC W. The methane fermentations of acetate and methanol. J Bacteriol. 1957 Jun;73(6):736–742. doi: 10.1128/jb.73.6.736-742.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romesser J. A., Balch W. E. Coenzyme M: preparation and assay. Methods Enzymol. 1980;67:545–552. doi: 10.1016/s0076-6879(80)67067-0. [DOI] [PubMed] [Google Scholar]
- STADTMAN T. C., BARKER H. A. Studies on the methane fermentation. IX. The origin of methane in the acetate and methanol fermentations by methanosarcina. J Bacteriol. 1951 Jan;61(1):81–86. doi: 10.1128/jb.61.1.81-86.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer F. D., Erfle J. D., Mahadevan S. Methane synthesis without the addition of adenosine triphosphate by cell membranes isolated from Methanobacterium ruminantium. Biochem J. 1979 Jan 15;178(1):165–172. doi: 10.1042/bj1780165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Smith M. R., Mah R. A. Growth and methanogenesis by Methanosarcina strain 227 on acetate and methanol. Appl Environ Microbiol. 1978 Dec;36(6):870–879. doi: 10.1128/aem.36.6.870-879.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R. Reversal of 2-bromoethanesulfonate inhibition of methanogenesis in Methanosarcina sp. J Bacteriol. 1983 Nov;156(2):516–523. doi: 10.1128/jb.156.2.516-523.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. H., Mah R. A. Kinetics of acetate metabolism during sludge digestion. Appl Microbiol. 1966 May;14(3):368–371. doi: 10.1128/am.14.3.368-371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J., Wolfe R. S. Complete degradation of carbohydrate to carbon dioxide and methane by syntrophic cultures of Acetobacterium woodii and Methanosarcina barkeri. Arch Microbiol. 1979 Apr;121(1):97–102. doi: 10.1007/BF00409211. [DOI] [PubMed] [Google Scholar]
- Zinder S. H., Mah R. A. Isolation and Characterization of a Thermophilic Strain of Methanosarcina Unable to Use H(2)-CO(2) for Methanogenesis. Appl Environ Microbiol. 1979 Nov;38(5):996–1008. doi: 10.1128/aem.38.5.996-1008.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



