Abstract
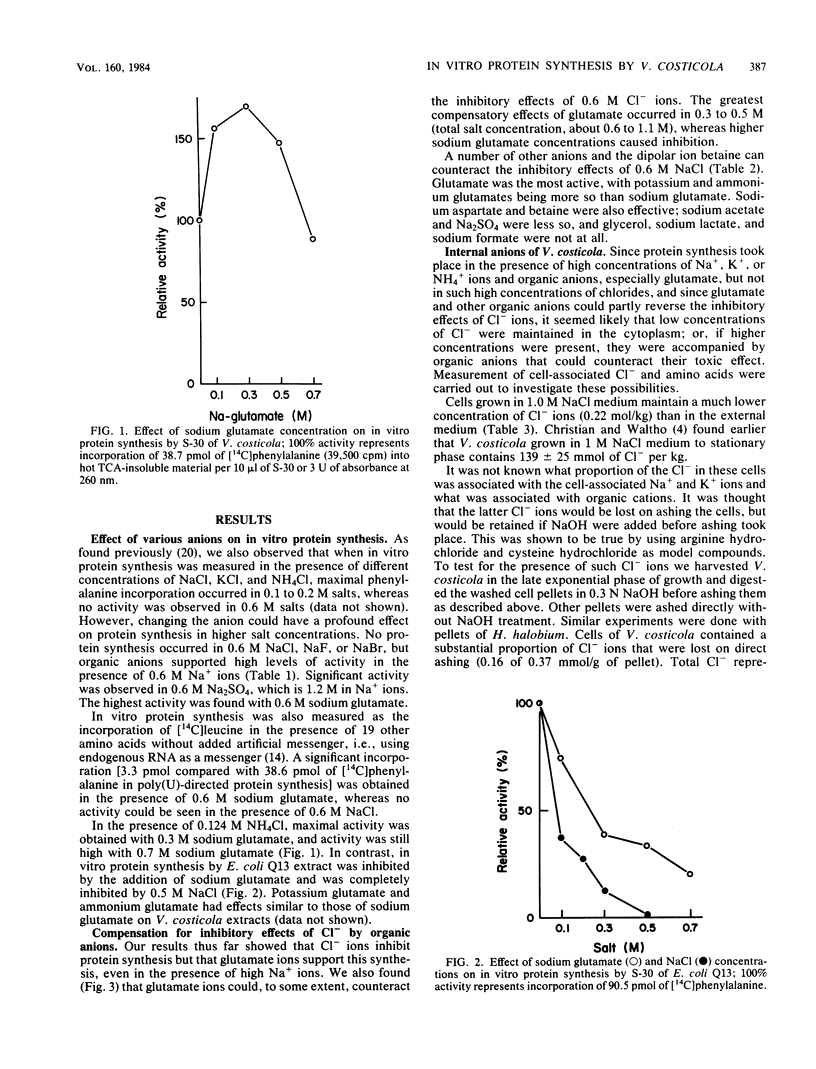
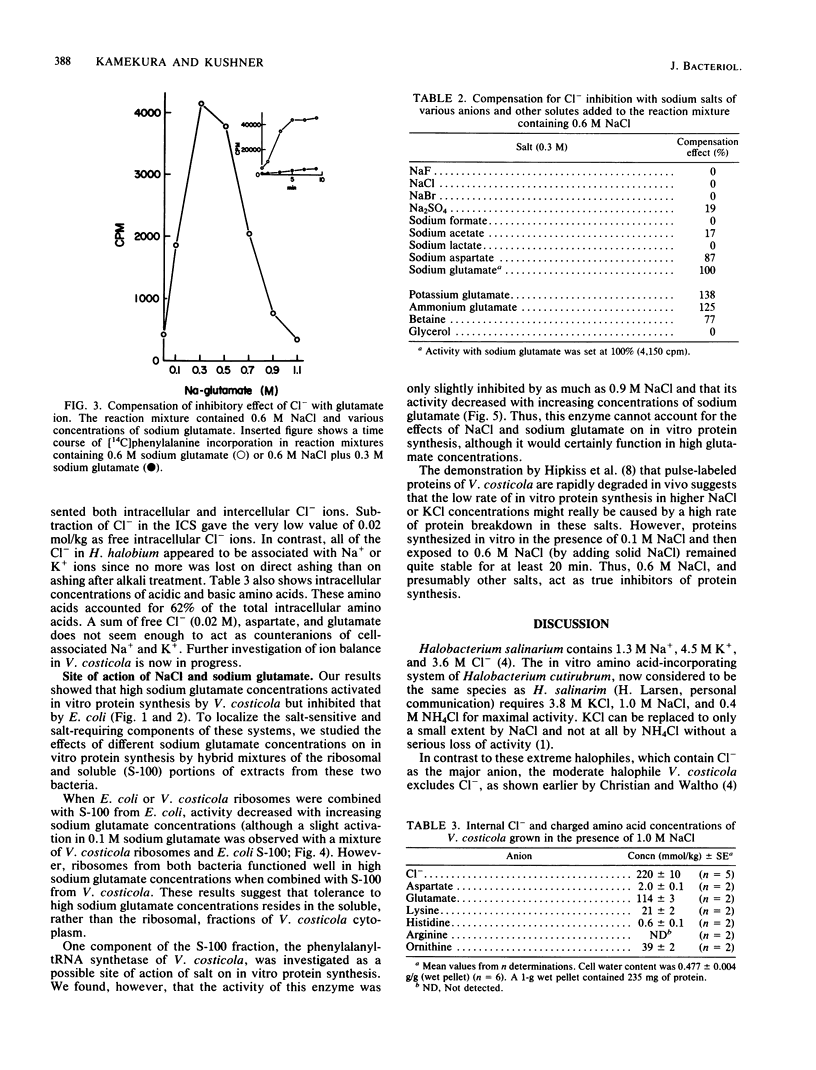
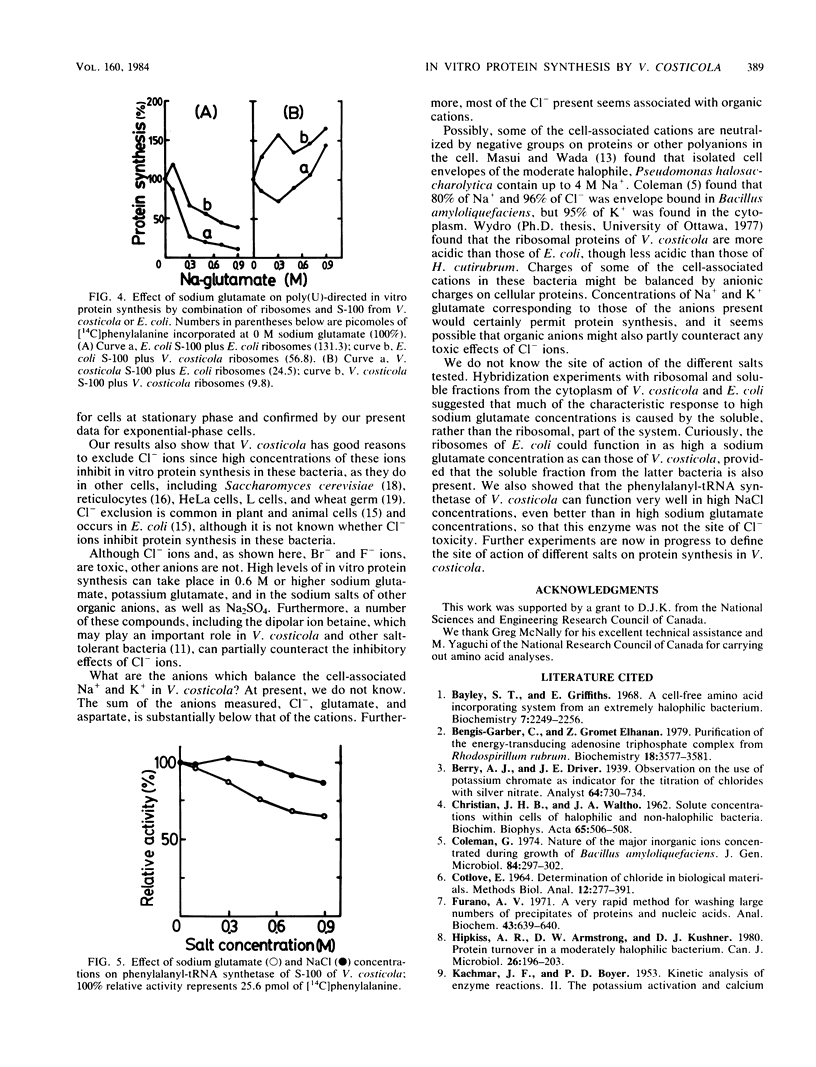
Vibrio costicola grown in the presence of different NaCl concentrations contains cell-associated Na+ and K+ ions whose sum is equal to or greater than the external Na+ concentration. In the presence of 0.5 M NaCl, virtually no in vitro protein is synthesized in extracts of cells grown in 1.0 M NaCl. However, we report here that active in vitro protein synthesis occurred in 0.6 M or higher concentrations of Na2SO4, sodium formate, sodium acetate, sodium aspartate, or sodium glutamate, whereas 0.6 M NaF, NaCl, or NaBr completely inhibited protein synthesis as measured by polyuridylic acid-directed incorporation of [14C]phenylalanine. Sodium glutamate, sodium aspartate, and betaine (0.3 M) counteracted the inhibitory action of 0.6 M NaCl. The cell-associated Cl- concentration was 0.22 mol/kg in cells grown in 1.0 M NaCl. Of this, the free intracellular Cl- concentration was only 0.02 mol/kg. Cells contained 0.11 mol of glutamate per kg and small concentrations of other amino acids. All of the negative counterions for cell-associated Na+ and K+ have not yet been determined. In vitro protein synthesis by Escherichia coli was inhibited by sodium glutamate. Hybridization experiments with ribosomes and the soluble (S-100) fractions from extracts of E. coli and V. costicola showed that the glutamate-sensitive fraction was found in the soluble, not the ribosomal, part of the system. The phenylalanyl-tRNA synthetase of V. costicola was not inhibited by 0.5 M or higher concentrations of NaCl; it was slightly more sensitive to high concentrations of sodium glutamate. Therefore, this enzyme was not responsible for the salt response of the V. costicola in vitro protein-synthesizing system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayley S. T., Griffiths E. A cell-free amino acid incorporating system from an extremely halophilic bacterium. Biochemistry. 1968 Jun;7(6):2249–2256. doi: 10.1021/bi00846a030. [DOI] [PubMed] [Google Scholar]
- Bengis-Garber C., Gromet-Elhanan Z. Purification of the energy-transducing adenosine triphosphatase complex from Rhodospirillum rubrum. Biochemistry. 1979 Aug 7;18(16):3577–3581. doi: 10.1021/bi00583a022. [DOI] [PubMed] [Google Scholar]
- CHRISTIAN J. H., WALTHO J. A. Solute concentrations within cells of halophilic and non-halophilic bacteria. Biochim Biophys Acta. 1962 Dec 17;65:506–508. doi: 10.1016/0006-3002(62)90453-5. [DOI] [PubMed] [Google Scholar]
- Coleman G. Nature of the major inorganic ions concentrated during growth of Bacillus amyloliquefaciens. J Gen Microbiol. 1974 Oct;84(2):297–302. doi: 10.1099/00221287-84-2-297. [DOI] [PubMed] [Google Scholar]
- Di Segni G., Rosen H., Kaempfer R. Competition between alpha- and beta-globin messenger ribonucleic acids for eucaryotic initiation factor 2. Biochemistry. 1979 Jun 26;18(13):2847–2854. doi: 10.1021/bi00580a027. [DOI] [PubMed] [Google Scholar]
- Furano A. V. A very rapid method for washing large numbers of precipitates of proteins and nucleic acids. Anal Biochem. 1971 Oct;43(2):639–640. doi: 10.1016/0003-2697(71)90300-9. [DOI] [PubMed] [Google Scholar]
- Hipkiss A. R., Armstrong D. W., Kushner D. J. Protein turnover in a moderately halophilic bacterium. Can J Microbiol. 1980 Feb;26(2):196–203. doi: 10.1139/m80-030. [DOI] [PubMed] [Google Scholar]
- Kushner D. J., Hamaide F., MacLeod R. A. Development of salt-resistant active transport in a moderately halophilic bacterium. J Bacteriol. 1983 Mar;153(3):1163–1171. doi: 10.1128/jb.153.3.1163-1171.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Masui M., Wada S. Intracellular concentrations of Na+, K+, and cl minus of a moderately halophilic bacterium. Can J Microbiol. 1973 Oct;19(10):1181–1186. doi: 10.1139/m73-191. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., WILSON N. L., EPSTEIN W. Cation transport in Escherichia coli. II. Intracellular chloride concentration. J Gen Physiol. 1962 Sep;46:159–166. doi: 10.1085/jgp.46.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindler D. B., Wydro R. M., Kushner D. J. Cell-bound cations of the moderately halophilic bacterium Vibrio costicola. J Bacteriol. 1977 May;130(2):698–703. doi: 10.1128/jb.130.2.698-703.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuite M. F., Plesset J., Moldave K., McLaughlin C. S. Faithful and efficient translation of homologous and heterologous mRNAs in an mRNA-dependent cell-free system from Saccharomyces cerevisiae. J Biol Chem. 1980 Sep 25;255(18):8761–8766. [PubMed] [Google Scholar]
- Weber L. A., Hickey E. D., Maroney P. A., Baglioni C. Inhibition of protein synthesis by Cl-. J Biol Chem. 1977 Jun 10;252(11):4007–4010. [PubMed] [Google Scholar]
- Wydro R. M., Madira W., Hiramatsu T., Kogut M., Kushner D. J. Salt-sensitive in vitro protein synthesis by a moderately halophilic bacterium. Nature. 1977 Oct 27;269(5631):824–825. doi: 10.1038/269824a0. [DOI] [PubMed] [Google Scholar]


