Abstract
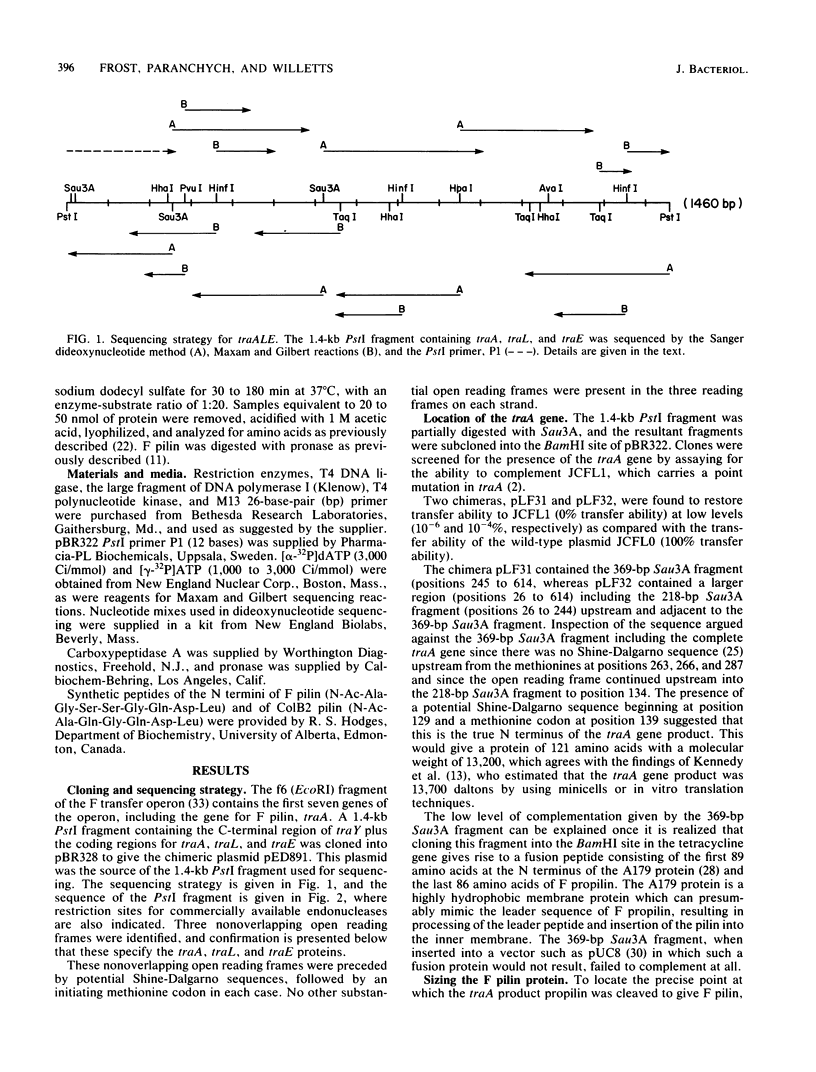
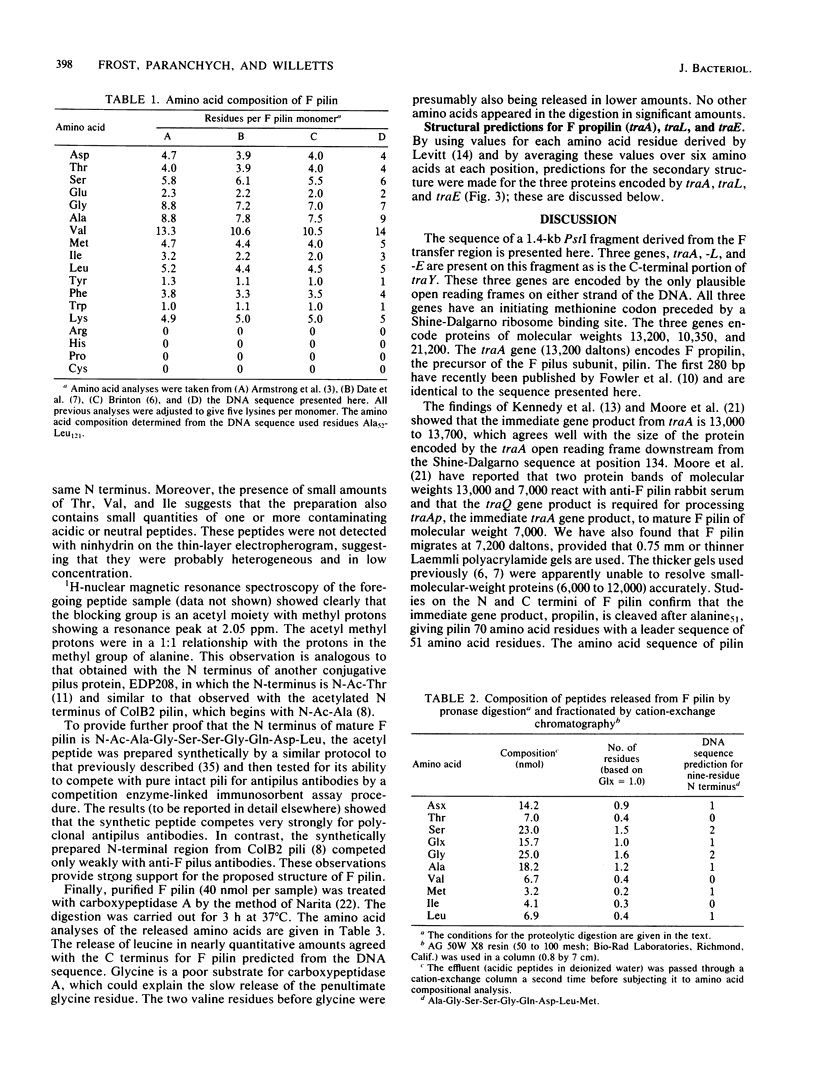
The complete sequence of a 1.4-kilobase PstI fragment containing the F transfer genes traA, -L, and -E is presented. The traA reading frame has been located both genetically and by comparing the primary structure of F pilin (the traA product) predicted by the DNA sequence to the amino acid composition and sequence of N- and C-terminal peptides isolated from purified F pilin. Taken together, these data show that there is a leader peptide of 51 amino acids and that F pilin contains 70 amino acids, giving molecular weights of 13,200 for F propilin and 7,200 for mature F pilin. Secondary structure predictions for F pilin revealed a reverse turn that precedes the sequence Ala-Met-Ala51, a classic signal peptidase cleavage site. The N-terminal alanine residue is blocked by an acetyl group as determined by 1H-nuclear magnetic resonance spectroscopy. The traL and traE genes encode proteins of molecular weights 10,350 and 21,200, respectively. According to DNA sequence predictions, these proteins do not contain signal peptide leader sequences. Secondary structure predictions for these proteins are in accord with traLp and traEp being membrane proteins in which hydrophobic regions capable of spanning the membrane are linked by sequences that form turns and carry positively charged residues capable of interacting with the membrane surface.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Manning P. A., Edelbluth C., Herrlich P. Export without proteolytic processing of inner and outer membrane proteins encoded by F sex factor tra cistrons in Escherichia coli minicells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4837–4841. doi: 10.1073/pnas.76.10.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Conjugational complementation analysis of transfer-deficient mutants of Flac in Escherichia coli. J Bacteriol. 1972 Jun;110(3):831–842. doi: 10.1128/jb.110.3.831-842.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong G. D., Frost L. S., Sastry P. A., Paranchych W. Comparative biochemical studies on F and EDP208 conjugative pili. J Bacteriol. 1980 Jan;141(1):333–341. doi: 10.1128/jb.141.1.333-341.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong G. D., Frost L. S., Vogel H. J., Paranchych W. Nature of the carbohydrate and phosphate associated with ColB2 and EDP208 pilin. J Bacteriol. 1981 Mar;145(3):1167–1176. doi: 10.1128/jb.145.3.1167-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The properties of sex pili, the viral nature of "conjugal" genetic transfer systems, and some possible approaches to the control of bacterial drug resistance. CRC Crit Rev Microbiol. 1971 May;1(1):105–160. doi: 10.3109/10408417109104479. [DOI] [PubMed] [Google Scholar]
- Date T., Inuzuka M., Tomoeda M. Purification and characterization of F pili from Escherichia coli. Biochemistry. 1977 Dec 13;16(25):5579–5585. doi: 10.1021/bi00644a030. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Frost L. S., Paranchych W. Localization, cloning, and sequence determination of the conjugative plasmid ColB2 pilin gene. J Bacteriol. 1984 Oct;160(1):402–407. doi: 10.1128/jb.160.1.402-407.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkhard W., Leonard K. R., Malsey S., Marvin D. A., Dubochet J., Engel A., Achtman M., Helmuth R. X-ray diffraction and electron microscope studies on the structure of bacterial F pili. J Mol Biol. 1979 May 15;130(2):145–160. doi: 10.1016/0022-2836(79)90423-6. [DOI] [PubMed] [Google Scholar]
- Fowler T., Taylor L., Thompson R. The control region of the F plasmid transfer operon: DNA sequence of the traJ and traY genes and characterisation of the traY leads to Z promoter. Gene. 1983 Dec;26(1):79–89. doi: 10.1016/0378-1119(83)90038-0. [DOI] [PubMed] [Google Scholar]
- Frost L. S., Armstrong G. D., Finlay B. B., Edwards B. F., Paranchych W. N-terminal amino acid sequencing of EDP208 conjugative pili. J Bacteriol. 1983 Feb;153(2):950–954. doi: 10.1128/jb.153.2.950-954.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. A., Willetts N. S. Construction and characterization of multicopy plasmids containing the entire F transfer region. Plasmid. 1980 Nov;4(3):292–304. doi: 10.1016/0147-619x(80)90068-2. [DOI] [PubMed] [Google Scholar]
- Kennedy N., Beutin L., Achtman M., Skurray R., Rahmsdorf U., Herrlich P. Conjugation proteins encoded by the F sex factor. Nature. 1977 Dec 15;270(5638):580–585. doi: 10.1038/270580a0. [DOI] [PubMed] [Google Scholar]
- Levitt M. Conformational preferences of amino acids in globular proteins. Biochemistry. 1978 Oct 3;17(20):4277–4285. doi: 10.1021/bi00613a026. [DOI] [PubMed] [Google Scholar]
- Lory S., Tai P. C., Davis B. D. Mechanism of protein excretion by gram-negative bacteria: Pseudomonas aeruginosa exotoxin A. J Bacteriol. 1983 Nov;156(2):695–702. doi: 10.1128/jb.156.2.695-702.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Jr, Polen S., Brinton C. C., Jr, Ippen-Ihler K. Identification of the structural gene for F-pilin. J Mol Biol. 1976 Nov;108(1):111–121. doi: 10.1016/s0022-2836(76)80098-8. [DOI] [PubMed] [Google Scholar]
- Moore D., Sowa B. A., Ippen-Ihler K. A new activity in the Ftra operon which is required for F-pilin synthesis. Mol Gen Genet. 1982;188(3):459–464. doi: 10.1007/BF00330049. [DOI] [PubMed] [Google Scholar]
- Moore D., Sowa B. A., Ippen-Ihler K. Location of an F-pilin pool in the inner membrane. J Bacteriol. 1981 Apr;146(1):251–259. doi: 10.1128/jb.146.1.251-259.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Quinn P. S., Chan S. J., Marsh J., Tager H. S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci. 1980;343:1–16. doi: 10.1111/j.1749-6632.1980.tb47238.x. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Thompson R., Taylor L. Promoter mapping and DNA sequencing of the F plasmid transfer genes traM and traJ. Mol Gen Genet. 1982;188(3):513–518. doi: 10.1007/BF00330058. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Suggs S. V., Miyoshi K., Bhatt R., Itakura K. A set of synthetic oligodeoxyribonucleotide primers for DNA sequencing in the plasmid vector pBR322. Gene. 1981 Dec;16(1-3):21–26. doi: 10.1016/0378-1119(81)90057-3. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Moore P. M., Paranchych W. Variant pili produced by mutants of the Flac plasmid. J Gen Microbiol. 1980 Apr;117(2):455–464. doi: 10.1099/00221287-117-2-455. [DOI] [PubMed] [Google Scholar]
- Willetts N., Skurray R. The conjugation system of F-like plasmids. Annu Rev Genet. 1980;14:41–76. doi: 10.1146/annurev.ge.14.120180.000353. [DOI] [PubMed] [Google Scholar]
- Worobec E. A., Taneja A. K., Hodges R. S., Paranchych W. Localization of the major antigenic determinant of EDP208 pili at the N-terminus of the pilus protein. J Bacteriol. 1983 Feb;153(2):955–961. doi: 10.1128/jb.153.2.955-961.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Blomberg C. Trans-membrane translocation of proteins. The direct transfer model. Eur J Biochem. 1979 Jun;97(1):175–181. doi: 10.1111/j.1432-1033.1979.tb13100.x. [DOI] [PubMed] [Google Scholar]


