Abstract
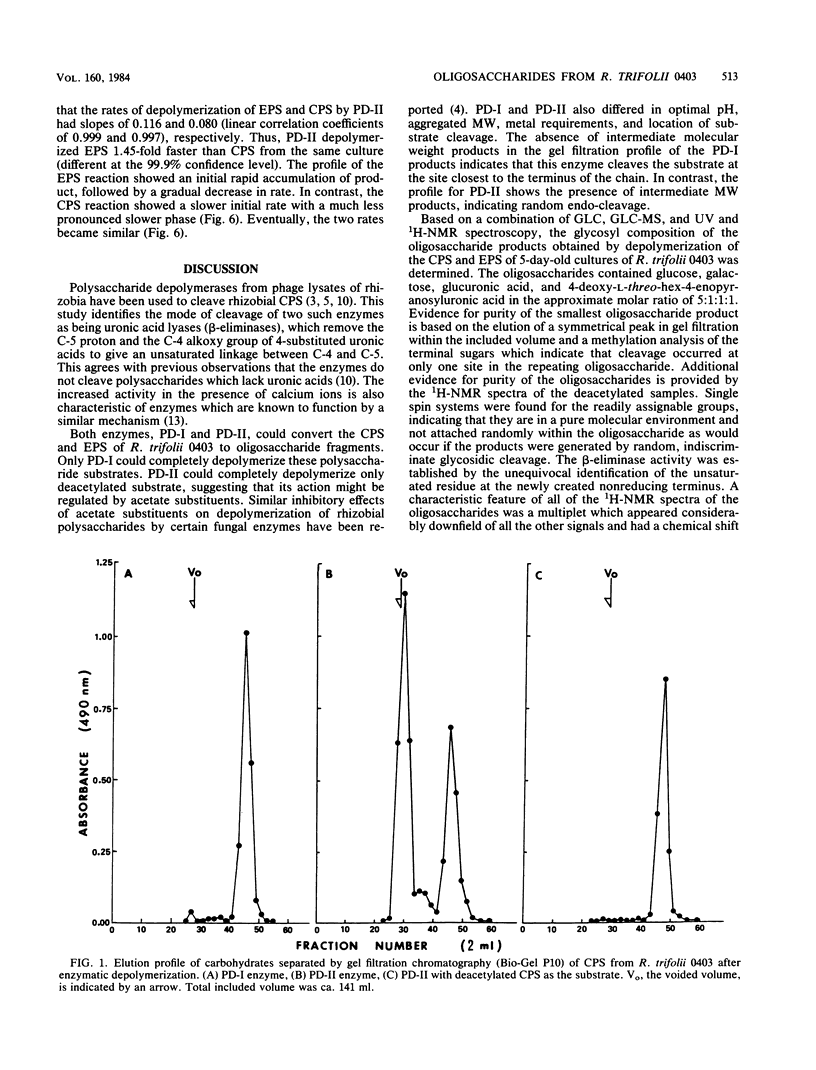
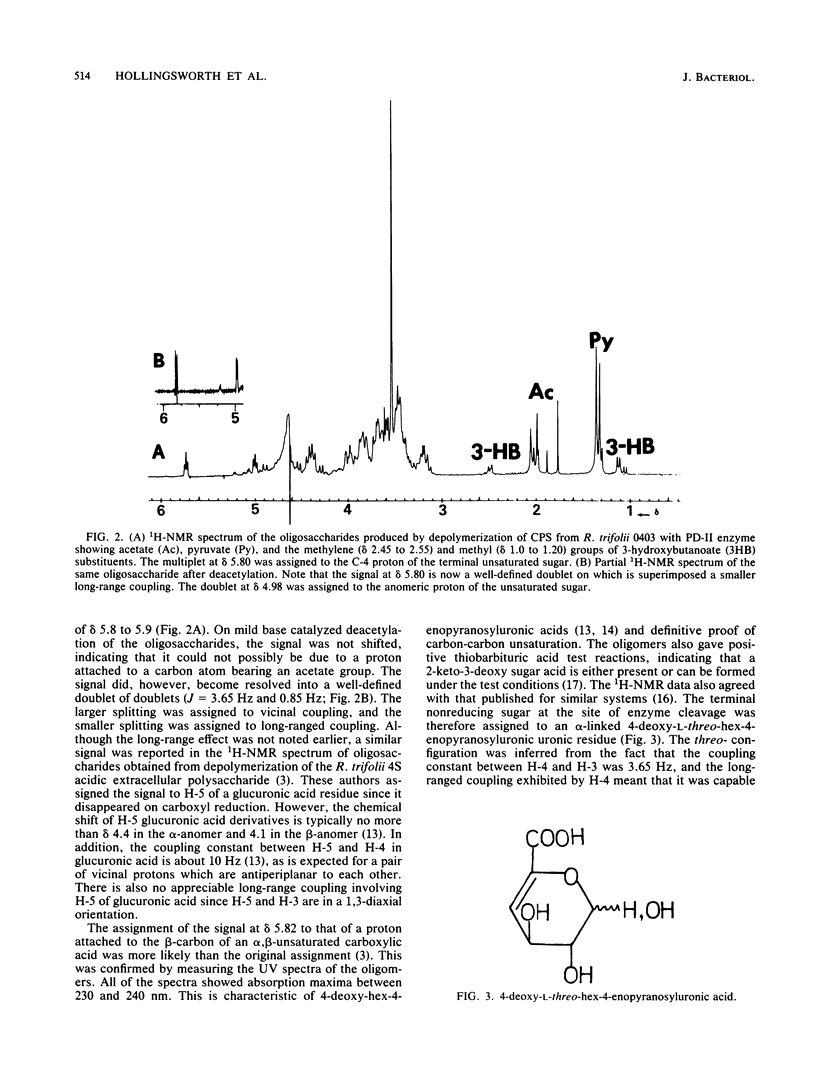
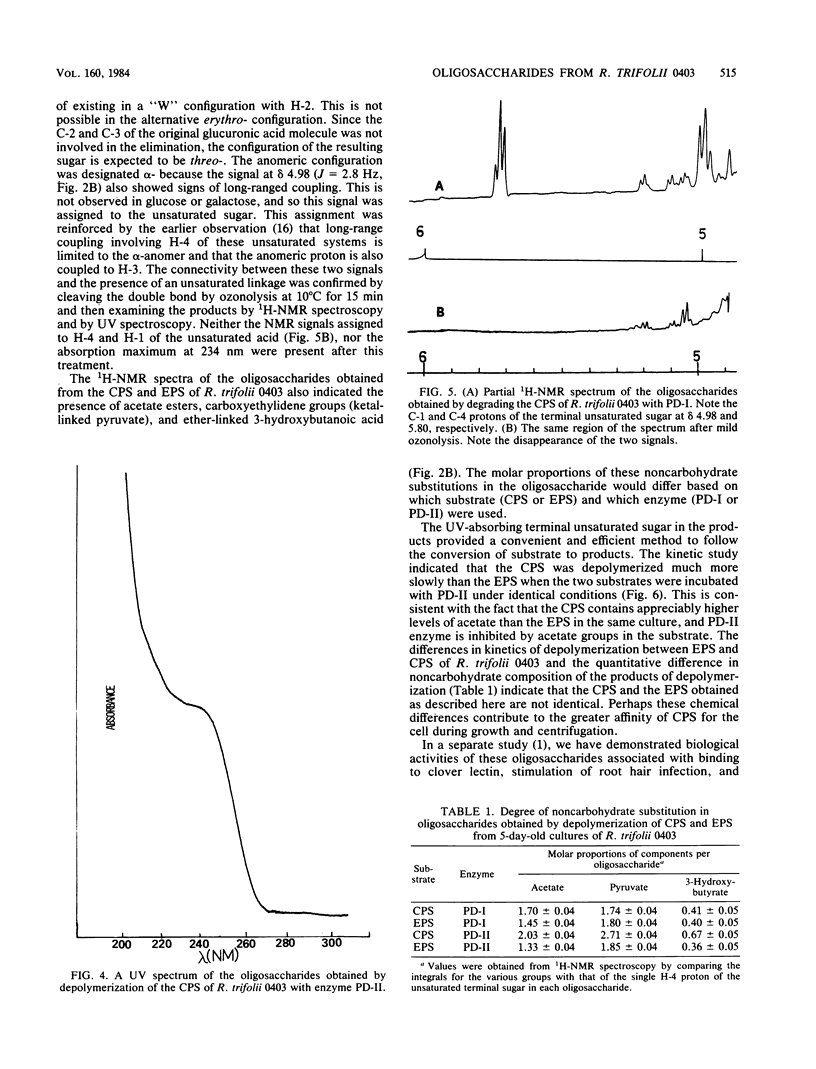
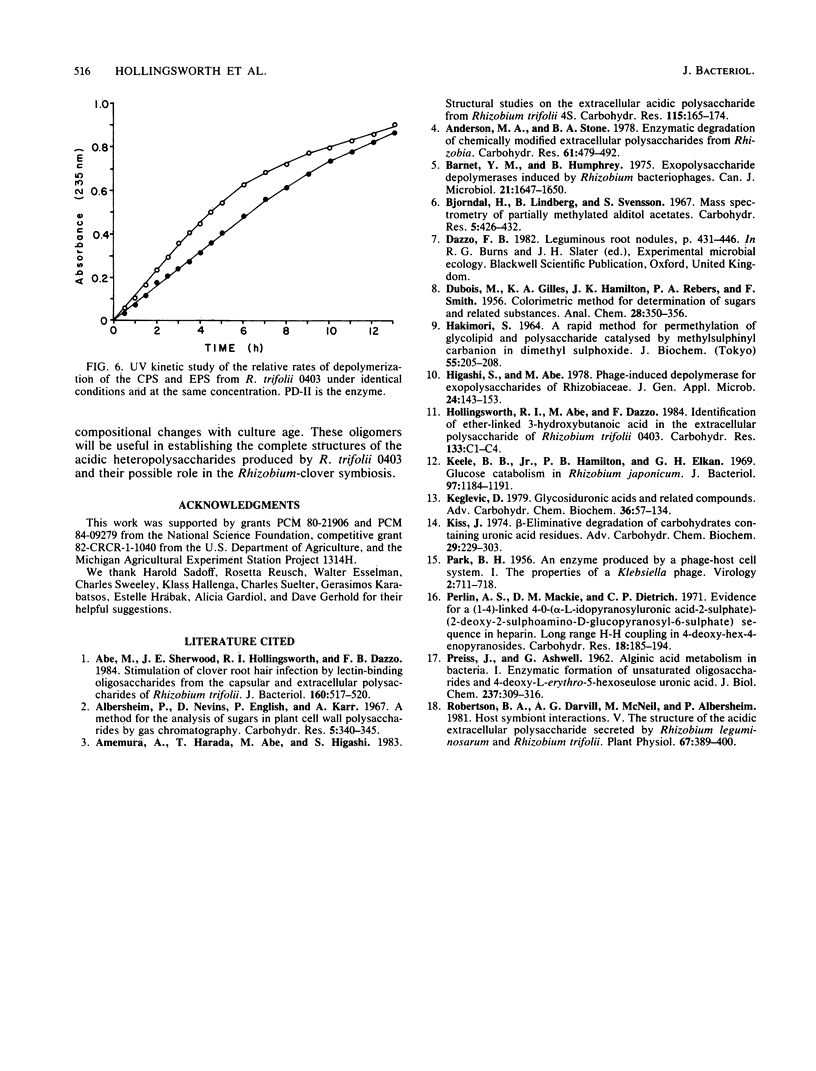
Acidic heteropolysaccharide lyases from lysates of phages 4S and BY15 grown on Rhizobium trifolii 4S and R. trifolii 0403, respectively, were used to analyze the capsular and excreted extracellular acidic polysaccharides of R. trifolii 0403. The activities of the enzymes as measured by viscometry were enhanced by the addition of calcium. The oligosaccharide products obtained by depolymerase digestion of the polysaccharides isolated from cells grown on agar plates for 5 days were isolated by gel filtration and had a glycosyl composition of glucose, galactose, glucuronic acid, and alpha-linked 4-deoxy-L-threo-hex-4-enopyranosyluronic acid in an approximate molar ratio of 5:1:1:1. This latter component was identified by 1H-nuclear magnetic resonance spectroscopy and confirmed by UV spectroscopy, ozonolysis, and its reactivity with thiobarbituric acid. The oligosaccharide had glucose as the reducing terminus, 4-deoxy-L-threo-hex-4-enopyranosyluronic acid as the enzymatically generated nonreducing terminus, and galactose as the terminus of the branched chain. The noncarbohydrate components of the oligosaccharides were acetate, ketal-linked pyruvate, and ether-linked 3-hydroxybutyrate. The mode of action of the enzymes was by beta-elimination from a uronic acid residue with concomitant loss of the glycosyl component substituted at C-4. The 235-nm absorbing properties of the resulting terminal unsaturated sugar were used to study the kinetics of depolymerization of the capsular and excreted extracellular acidic polysaccharides, using the enzyme from phage BY15. The two substrates exhibited different kinetics of depolymerization, and the oligosaccharide products differed in the amount of noncarbohydrate substituents, indicating that the acidic capsular and excreted extracellular polysaccharides from 5-day-old cultures of R. trifolii 0403 were different.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnet Y. M., Humphrey B. Exopolysaccharide depolymerases induced by Rhizobium bacteriophages. Can J Microbiol. 1975 Oct;21(10):1647–1650. doi: 10.1139/m75-239. [DOI] [PubMed] [Google Scholar]
- Keele B. B., Jr, Hamilton P. B., Elkan G. H. Glucose catabolism in Rhizobium japonicum. J Bacteriol. 1969 Mar;97(3):1184–1191. doi: 10.1128/jb.97.3.1184-1191.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK B. H. An enzyme produced by a phage-host cell system. I. The properties of a Klebsiella phage. Virology. 1956 Dec;2(6):711–718. doi: 10.1016/0042-6822(56)90053-8. [DOI] [PubMed] [Google Scholar]
- PREISS J., ASHWELL G. Alginic acid metabolism in bacteria. I. Enzymatic formation of unsaturated oligosac-charides and 4-deoxy-L-erythro-5-hexoseulose uronic acid. J Biol Chem. 1962 Feb;237:309–316. [PubMed] [Google Scholar]
- Perlin A. S., Mackie D. M., Dietrich C. P. Evidence for a (1 leads to 4)-linked 4-O-( -L-idopyranosyluronic acid 2-sulfate)-(2-deoxy-2-sulfoamino-D-glucopyranosyl 6-sulfate) sequence in heparin. Long-range H-H coupling in 4-deoxy-hex-4-enopyranosides. Carbohydr Res. 1971 Jun;18(2):185–194. doi: 10.1016/s0008-6215(00)80341-9. [DOI] [PubMed] [Google Scholar]
- Robertsen B. K., Aman P., Darvill A. G., McNeil M., Albersheim P. Host-Symbiont Interactions : V. THE STRUCTURE OF ACIDIC EXTRACELLULAR POLYSACCHARIDES SECRETED BY RHIZOBIUM LEGUMINOSARUM AND RHIZOBIUM TRIFOLII. Plant Physiol. 1981 Mar;67(3):389–400. doi: 10.1104/pp.67.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]


