Abstract
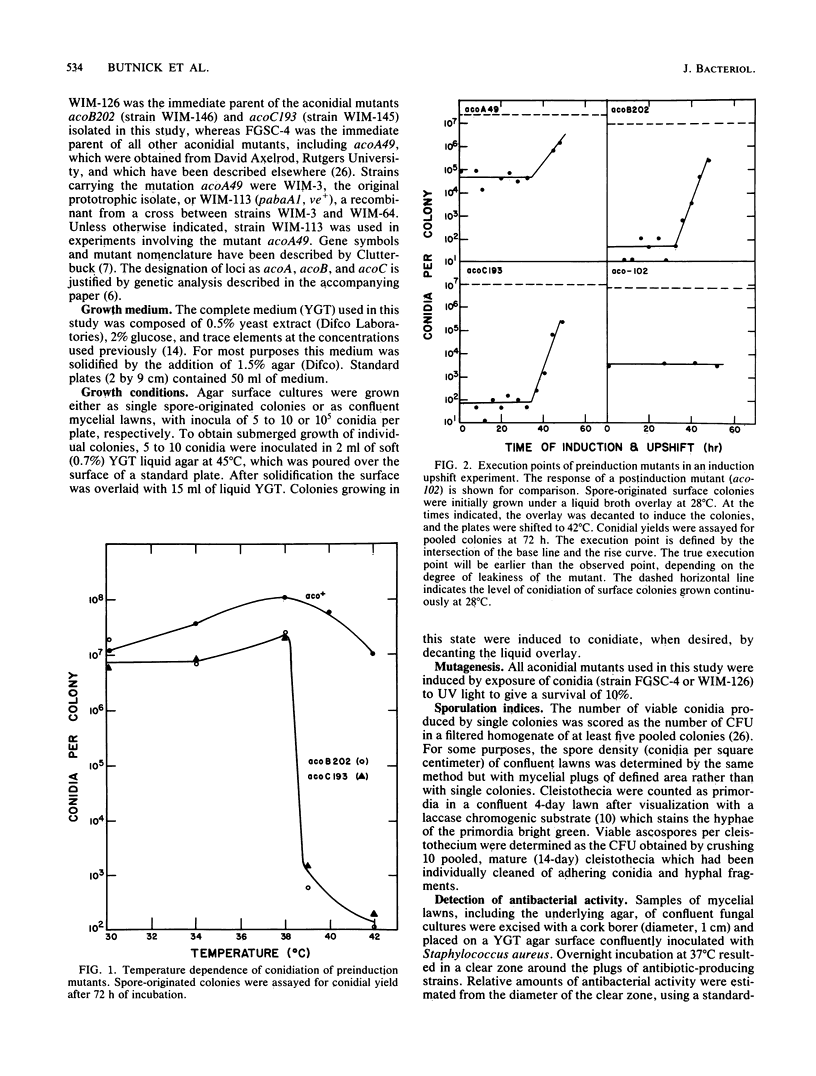
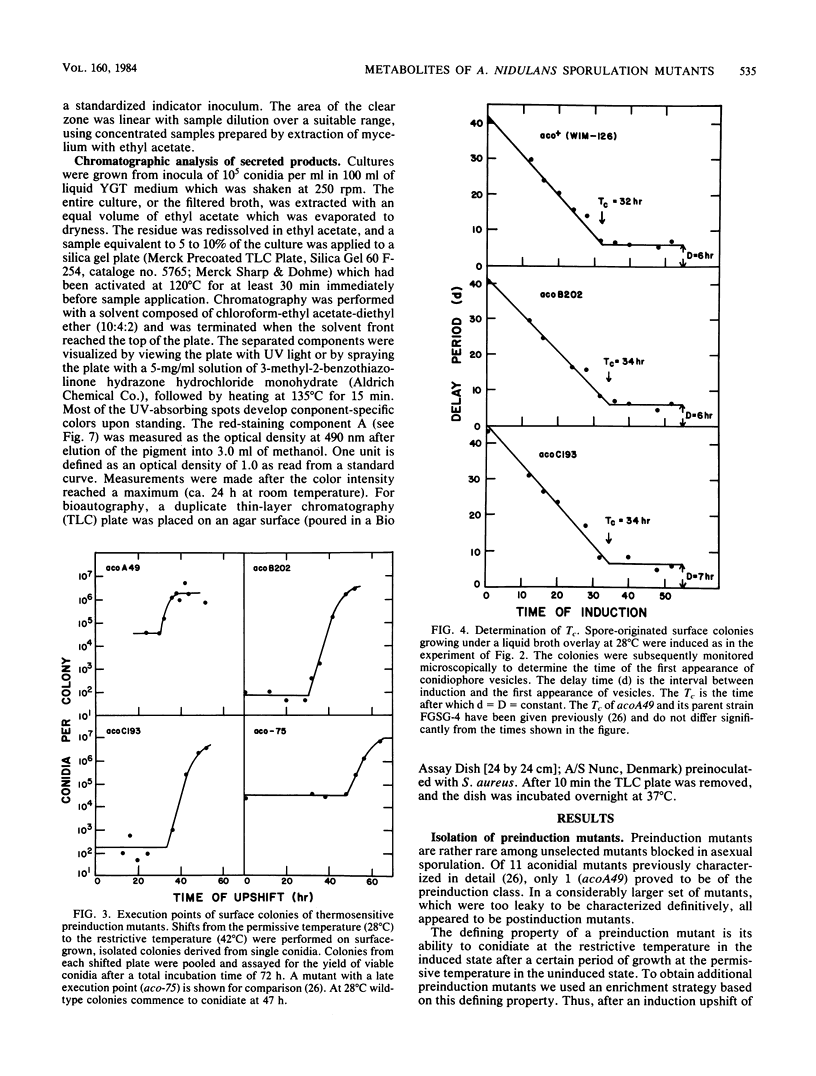
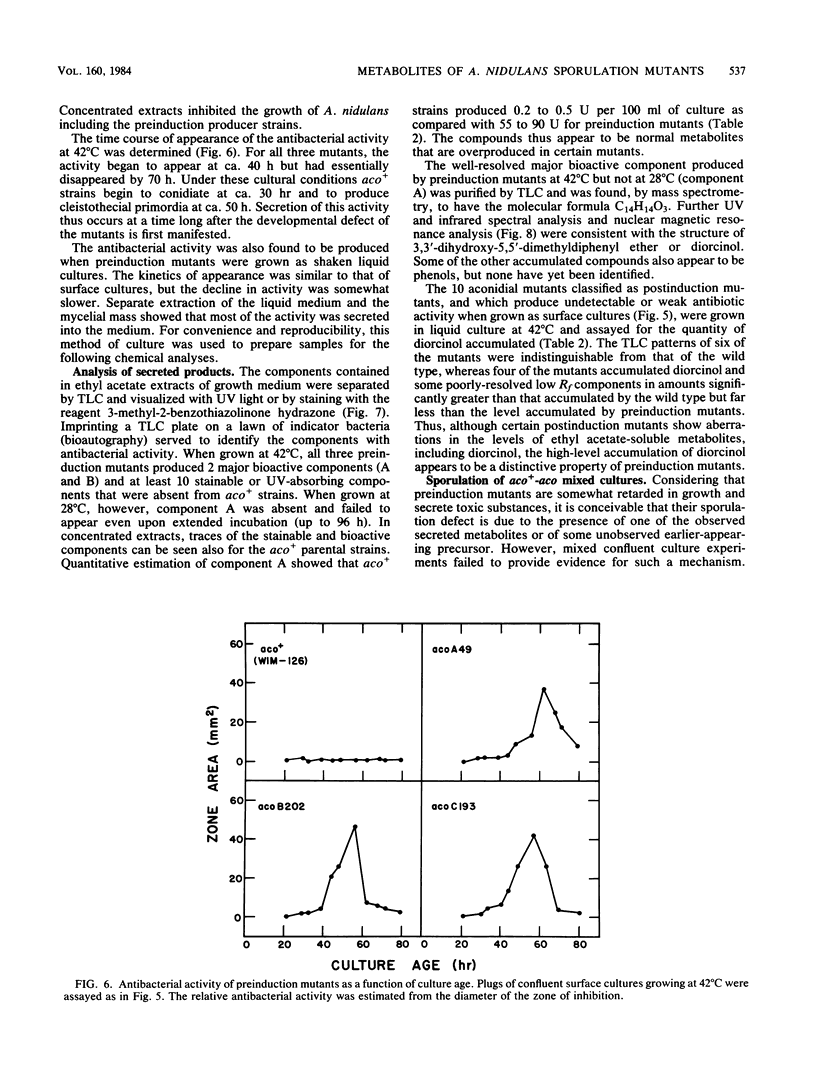
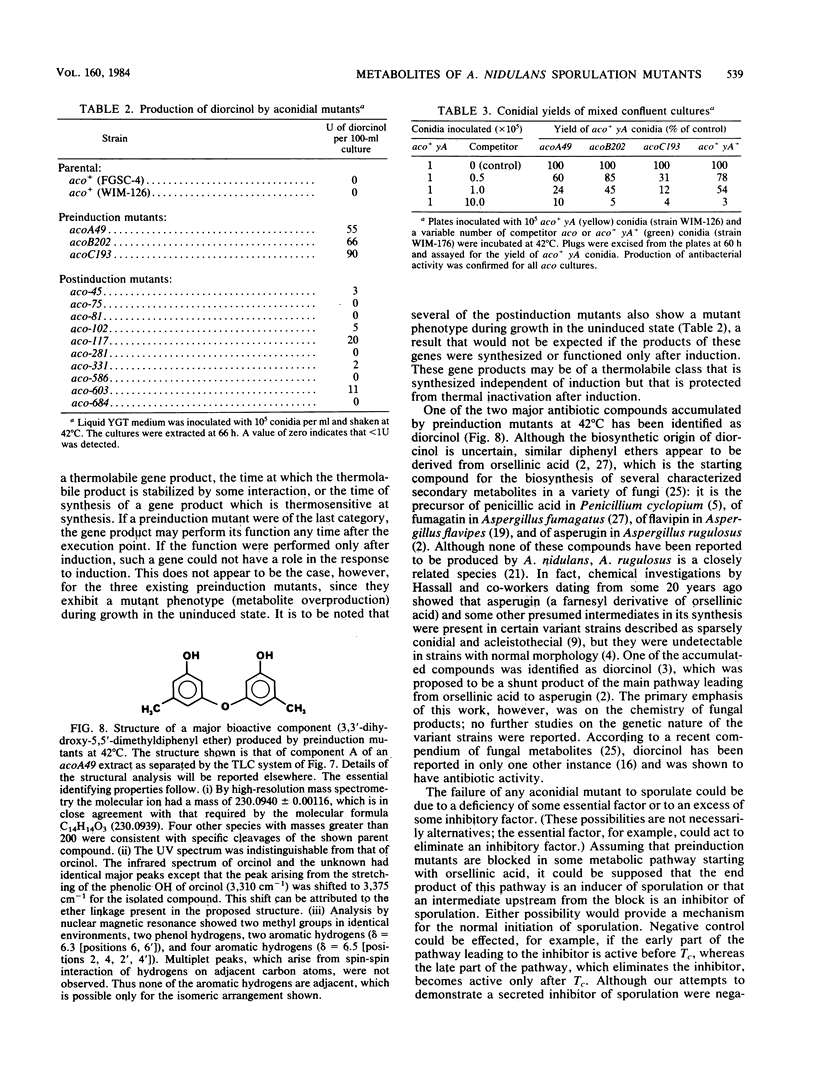
Mutants of Aspergillus nidulans defective in conidiation (asexual sporulation) can be classified according to whether they are blocked before or after induction of conidiation. Mutants blocked before induction (preinduction mutants) appear to be unable to respond to the inducing stimulus and thus are defective in one of the earliest events in the sporulation process. Three preinduction mutants have been isolated and characterized. Each was found to exhibit the same pleiotropic phenotype: they also were defective in sexual sporulation and secreted a set of phenolic metabolites at a level much higher than did wild type or mutants blocked at later stages of conidiation. One of the metabolites has been identified as the antibiotic diorcinal (3,3'-dihydroxy-5,5'-dimethyldiphenyl ether) which is known to be involved in the synthesis of certain farnesyl phenols of unknown function. These results suggest that preinduction mutants are blocked in a phenolic metabolic pathway, one or more product of which participates in the initiation of sporulation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D. E., Gealt M., Pastushok M. Gene control of developmental competence in Aspergillus nidulans. Dev Biol. 1973 Sep;34(1):9–15. doi: 10.1016/0012-1606(73)90335-7. [DOI] [PubMed] [Google Scholar]
- BENTLEY R., KEIL J. G. Tetronic acid biosynthesis in molds. II. Formation of penicillic acid in Penicillium cyclopium. J Biol Chem. 1962 Mar;237:867–873. [PubMed] [Google Scholar]
- Ballantine J. A., Hassall C. H., Jones G. The biosynthesis of phenols. IX. Asperugin, a metabolic product of Aspergillus rugulosus. J Chem Soc Perkin 1. 1965 Sep;:4672–4678. doi: 10.1039/jr9650004672. [DOI] [PubMed] [Google Scholar]
- HASSALL C. H., LAWRENCE K. THE RELATIONSHIP OF A PHENOLIC METABOLITE AND A MORPHOLOGICAL CHARACTER OF ASPERGILLUS RUGULOSUS. J Gen Microbiol. 1964 Jun;35:483–489. doi: 10.1099/00221287-35-3-483. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Culotti J., Reid B. Genetic control of the cell-division cycle in yeast. I. Detection of mutants. Proc Natl Acad Sci U S A. 1970 Jun;66(2):352–359. doi: 10.1073/pnas.66.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann T. E., Kurtz M. B., Champe S. P. Laccase localized in hulle cells and cleistothecial primordia of Aspergillus nidulans. J Bacteriol. 1983 May;154(2):955–964. doi: 10.1128/jb.154.2.955-964.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A. Genetics of bacterial sporulation. Adv Genet. 1976;18:69–98. doi: 10.1016/s0065-2660(08)60437-x. [DOI] [PubMed] [Google Scholar]
- Jarvik J., Botstein D. A genetic method for determining the order of events in a biological pathway. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2046–2050. doi: 10.1073/pnas.70.7.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. C., Moran C. P., Jr, Losick R. Two RNA polymerase sigma factors from Bacillus subtilis discriminate between overlapping promoters for a developmentally regulated gene. Nature. 1983 Apr 28;302(5911):800–804. doi: 10.1038/302800a0. [DOI] [PubMed] [Google Scholar]
- Kurtz M. B., Champe S. P. Dominant spore color mutants of Aspergillus nidulans defective in germination and sexual development. J Bacteriol. 1981 Nov;148(2):629–638. doi: 10.1128/jb.148.2.629-638.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Pero J. Cascades of Sigma factors. Cell. 1981 Sep;25(3):582–584. doi: 10.1016/0092-8674(81)90164-1. [DOI] [PubMed] [Google Scholar]
- Nair M. S., Carey S. T. Metabolites of pyrenomycetes: XII. Polyketides from the Hypocreales. Mycologia. 1979 Sep-Oct;71(5):1089–1096. [PubMed] [Google Scholar]
- Ollington J. F., Haldenwang W. G., Huynh T. V., Losick R. Developmentally regulated transcription in a cloned segment of the Bacillus subtilis chromosome. J Bacteriol. 1981 Aug;147(2):432–442. doi: 10.1128/jb.147.2.432-442.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastushok M., Axelrod D. E. Effect of glucose, ammonium and media maintenance on the time of conidiophore initiation by surface colonies of Aspergillus nidulans. J Gen Microbiol. 1976 May;94(1):221–224. doi: 10.1099/00221287-94-1-221. [DOI] [PubMed] [Google Scholar]
- Pettersson G. The biosynthesis of flavipin. II. Incorporation of aromatic precursors. Acta Chem Scand. 1965;19(7):1724–1732. doi: 10.3891/acta.chem.scand.19-1724. [DOI] [PubMed] [Google Scholar]
- Piggot P. J., Coote J. G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976 Dec;40(4):908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow H. The peptide antibiotic gramicidin D. A specific reactivator of tyrocidine-inhibited transcription. Biochim Biophys Acta. 1977 Jul 15;477(2):177–184. doi: 10.1016/0005-2787(77)90233-7. [DOI] [PubMed] [Google Scholar]
- Sarkar N., Langley D., Paulus H. Biological function of gramicidin: selective inhibition of RNA polymerase. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1478–1482. doi: 10.1073/pnas.74.4.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake W. E. Developmental gene regulation in Aspergillus nidulans. Dev Biol. 1980 Aug;78(2):497–510. doi: 10.1016/0012-1606(80)90349-8. [DOI] [PubMed] [Google Scholar]
- Yager L. N., Kurtz M. B., Champe S. P. Temperature-shift analysis of conidial development in Aspergillus nidulans. Dev Biol. 1982 Sep;93(1):92–103. doi: 10.1016/0012-1606(82)90242-1. [DOI] [PubMed] [Google Scholar]
- Zimmermann C. R., Orr W. C., Leclerc R. F., Barnard E. C., Timberlake W. E. Molecular cloning and selection of genes regulated in Aspergillus development. Cell. 1980 Oct;21(3):709–715. doi: 10.1016/0092-8674(80)90434-1. [DOI] [PubMed] [Google Scholar]




