Abstract
Several recent papers have been critical at a theoretical and empirical level of the evidence of population-level right-handedness in chimpanzees and other great apes. For example, Palmer (2002) has recently argued that the evidence of population-level handedness in chimpanzees is weak because there are sampling biases in the data. McGrew and Marchant (1997) argue that all the evidence of right-handedness in apes is from captive animals and therefore the observed phenomenon has little ecological validity. In this paper, we address recent issues regarding the presentation and interpretation of other hand preference data and argue that chimpanzees are right-handed for some measures. We further argue that purported differences in hand use between wild and captive chimpanzees due to rearing environments are unfounded and we emphasise that more cooperative work between researchers working in captive and feral populations is needed to facilitate collection of data on common measures of hand preference.
The study of handedness in nonhuman primates has recently received a great deal of attention in the scientific community. Historically, it was largely thought that nonhuman primate handedness was bimodally distributed with equal numbers of left- and right-handed individuals (see Bradshaw & Rogers, 1993; Fagot & Vauclair, 1991; Hook-Costigan & Rogers, 1997; Hopkins, 1996; Hopkins & Morris, 1993; Lehman, 1993; Marchant & McGrew, 1991). This distribution was in contrast to human handedness, which was skewed towards population-level right-handedness. Recent studies in a host of nonhuman primate species have reported population-level left- and right-handedness for select measures of hand use, calling into question the historical views regarding differences in the distributions of nonhuman and human primate handedness (Bradshaw & Rogers, 1993; Ward & Hopkins, 1993).
Despite the evidence of population-level handedness in nonhuman primates, there remains continued debate over the analysis and interpretation of findings on handedness, particularly in great apes (Hopkins, 1999; McGrew & Marchant, 1997). Specifically, Palmer (2002) has recently questioned the analysis and interpretation of findings on hand preference in nonhuman primates, and in particular, results suggesting population-level right-handedness in chimpanzees. Specifically, Palmer (2002) re-analysed hand preference data for bimanual feeding in chimpanzees reported by Hopkins (1994) and argued, based on his analysis using funnel graphs, that there were unusual patterns in the data that raised questions of the validity of the results for these data. Palmer (2002) went on to further argue, after re-analysis of 14 select studies on hand preference in chimpanzees, that there were limitations for nearly all of the articles, and from this he argued that there was no compelling evidence of population-level handedness in chimpanzees, and perhaps other great apes.
With respect to the findings reported by Hopkins (1994), Palmer (2002) argued that a fundamental problem with the data was in the relationship between the number of individual data points collected for each subject and the distribution of hand preferences in the sample. Palmer (2002) quartiled the sample (N = 140) on the basis of the total number of observations used to derive the individual handedness scores (see his Table 1) and showed that as the number of observation increased, the number of ambiguously handed subjects increased. In short, the data regressed towards a mean of zero, or no hand preference, with increasing sample size. Palmer (2002) also created funnel graphs of the individual variation in percent right hand use as a function of sample size and argued that the patterns did not conform to patterns that would normally reflect simple sampling error in the Hopkins (1994) data. Lastly, Palmer (2002) applied funnel graph analysis to 14 studies on hand preference in chimpanzees selectively used by McGrew and Marchant (1997) in their review article and argued that most studies found no evidence of population-level handedness and that all the evidence of population-level right-handedness came from studies by Hopkins and colleagues. Palmer (2002) raises questions regarding the generality of the results.
TABLE 1.
Distribution of hand preference for five behaviours recorded in captive and field studies
|
Captive |
Wild |
|||||
|---|---|---|---|---|---|---|
| #L | #R | #A | #L | #R | #A | |
| Scratch1 | 2 | 5 | 1 | 14 | 23 | 44 |
| Groom2 | 4 | 4 | 24 | 16 | 29 | 40 |
| Eat3 | 15 | 9 | 36 | 21 | 14 | 44 |
| Pick-up4 | 51 | 52 | 86 | 27 | 17 | 29 |
| Hold5 | 36 | 6 | 81 | 19 | 25 | 11 |
L = Left, A = Ambiguous, R = Right.
Data from Marchant & McGrew, 1996; McGrew & Marchant, 2001; Leavens, Aureli, Hopkins, & Hyatt, 2001.
Data from Boesch, 1991; Marchant & McGrew, 1996; McGrew & Marchant, 2001; Marchant, 1983; Steiner, 1990.
Data from Boesch, 1991; Marchant & McGrew, 1996; McGrew & Marchant, 2001; Sugiyama et al., 1993; Hopkins, 1993 (quadrupedal only); Marchant, 1983 (nonsocial reach only); Heestand, 1986; Steiner, 1990; Tonooka & Matsuzawa, 1995; Colell et al., 1995.
Although Palmer (2002) was critical of a specific set of data, more general concerns about the distribution and interpretation of hand preference findings in great apes have been articulated by McGrew and Marchant (1997). Although McGrew and Marchant (1997) have not been methodologically critical of any specific studies, they have questioned the generality of findings on hand preference in captive great apes to findings in wild subjects. McGrew and Marchant (1997) argue that the slight bias towards right-handedness reported in captive apes is due to the subjects being raised in a human environment and that wild apes do not show evidence of population-level handedness.
The purpose of this paper is two-fold. First, we begin by addressing the issue raised by Palmer (2002) regarding the application and use of funnel plots in the assessment of hand preference in chimpanzees (and other primates). We also present funnel graphs of additional sets of hand preference data collected in the chimpanzees housed at the Yerkes National Primate Research Center (YNPRC) which do not display the types of anomolous patterns that Palmer believes may characterise other sets of published data. Second, we discuss several other issues pertaining to the comparative analysis and interpretation of handedness findings in great apes compared to humans. In particular, we address in two ways issues of the generality of these findings to wild chimpanzees raised by McGrew and Marchant (1997). First, the issue of differences in the distribution of hand preference between wild and captive apes is empirically addressed by comparing findings on identical or similar measures of hand use that have been reported in wild and captive chimpanzees. Second, hand preferences on common measures of hand use are compared between wild chimpanzees from different field sites in Africa. The working hypothesis, based on the arguments of McGrew and Marchant (1997), is that variation between captive and wild populations of chimpanzees should be greater than variation between chimpanzees from different field sites in Africa.
FUNNEL GRAPHS AND HANDEDNESS
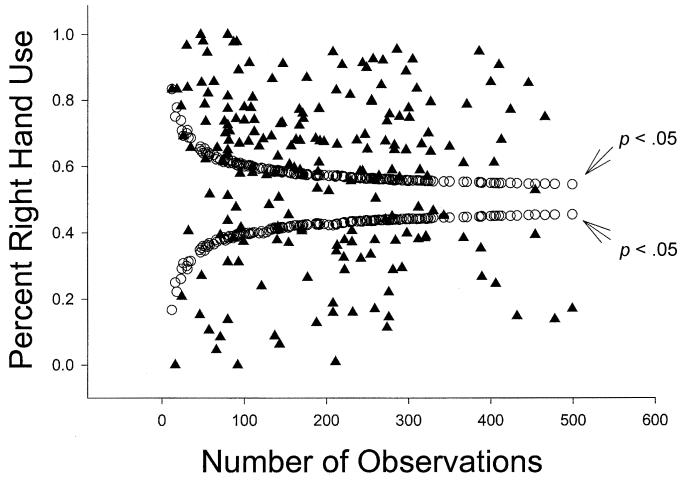
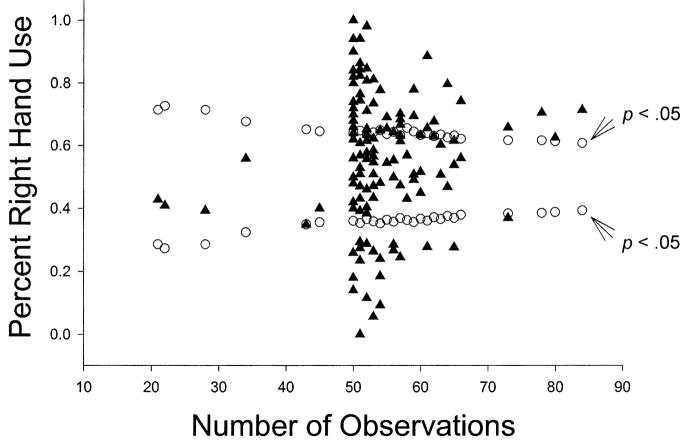
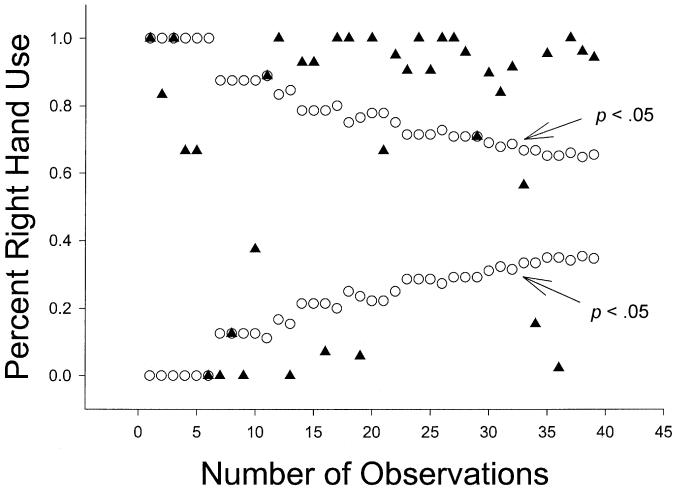
Palmer (2002) applied the funnel graph analysis to a single study on bimanual feeding in the chimpanzees and questioned all the results based on this analysis. The extent to which his application and findings using funnel graphs apply to other data from the YNPRC has not been directly examined. Depicted in Figures 1, 2, and 3, are funnel graphs of three separate data sets from the chimpanzees housed at the YNPRC. Figure 1 represents the combined TUBE data from Hopkins (1995), Hopkins, Fernandez-Carriba, Wesley, Hostetter, Pilcher, and Poss (2001) and Hopkins and Cantalupo (2003). Collectively, there were 202 chimpanzees tested on the TUBE task and the range of responses was between 12 and 500, respectively. Figure 2 represents simple reaching data from a sample of 140 chimpanzees, and the range of responses was between 23 and 79, with a large number of subjects with exactly 50 responses. Lastly, Figure 3 represents data for throwing from a sample of 39 chimpanzees with a range of responses between 6 and 77.
Figure 1.
Funnel plot of % right-handed use and sample size for the TUBE data summed for all studies at the YNPRC (N = 202).
Figure 2.
Funnel plot of % right-handed use and sample size for simple reaching data (N = 140).
Figure 3.
Funnel plot of % right-handed use and sample size for throwing data (N = 39).
We believe these data conform fairly well with the simulation funnel graphs representing different hypothetical hand preference distributions provided in the Palmer (2002) paper (see his Figure 1) because there are statistically significant left- and right-handed subjects across the spectrum of sample sizes in each distribution. Of particular note in Figures 1 and 3 is the lack of data points within the funnel portion of the distribution. This pattern emerges for two reasons. First, for the TUBE data depicted in Figure 1, the data were summed from three different reports and thus sample size was significantly increased at the individual level. This resulted in fewer ambiguously handed subjects and more subjects showing lateralised hand preferences. Second, for the throwing data depicted in Figure 3, chimpanzees show very pronounced hand preferences at the individual level, irrespective of sample size. In other words, hand use is much more uniform with many subjects showing exclusive or nearly exclusive left or right hand use. This highlights the importance of finding and quantifying reliable measures of hand preference that are sensitive enough to detect consistent hand preferences at the individual level. We will return to this point later in the discussion.
The extent to which the hand preference findings in the YNPRC chimpanzees generalise to other great apes, other samples of chimpanzees and to chimpanzees in the wild compared to captivity are now discussed. Palmer (2002, 2003) and others (McGrew & Marchant, 1997) have argued that the evidence of population-level right-handedness appears to be unique to captive apes, and particularly those housed at the YNPRC. The suggestion has been made that rearing apes in a human environment somehow causes them to develop right-handedness through some type of social learning mechanism or some unknown factor (cf. McGrew & Marchant, 2001). In the following sections, we argue against these claims.
IS THE EVIDENCE OF POPULATION-LEVEL RIGHT-HANDEDNESS RESTRICTED TO THE CAPTIVE YNPRC CHIMPANZEES?
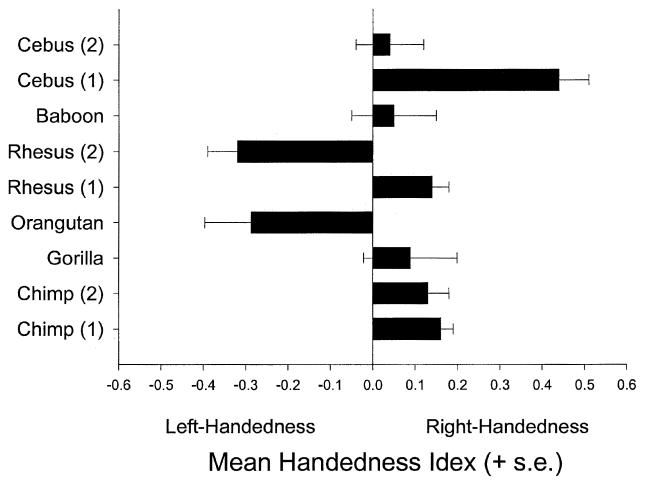
Palmer (2002) suggests that the strongest evidence of right-handedness in great apes has come from the YNPRC colony of chimpanzees, and specifically, from studies by Hopkins and colleagues. One interpretation is that the methods employed by Hopkins and colleagues are not objective enough and lead to an over-estimation of right-handedness by the observers. Of course, it could just as easily be argued that the measure(s) employed by Hopkins and colleagues are more sensitive to detecting individual and population-level handedness than measures employed in different laboratories or in field settings. However, we would go even further and argue that the evidence of population-level right-handedness is not restricted to the findings from the YNPRC colony. For example, using the TUBE task as a measure of comparison between captive species, population-level handedness has been reported in rhesus monkeys and, to a lesser extent, in capuchin monkeys (Spinozzi, Castornina, & Truppa, 1998; Westergaard & Suomi, 1996; Westergaard, Champoux, & Suomi, 1997). In other great apes, data from 31 gorillas suggest borderline right-handedness and significant population-level left-handedness in 19 orangutans (Hopkins, Stoinski, Lukas, Ross, & Wesley, 2003b). Lastly, TUBE data from 116 chimpanzees at the University of Texas M.D. Anderson Cancer Center (Hopkins, Hook, Braccini, & Schapiro, 2003a) and 148 chimpanzees at the Alamogordo Primate Facility in New Mexico show population-level right handedness (Hopkins, Wesley, Izard, Hook, & Schapiro, in press). The mean HI scores for each population can be seen in Figure 4. In short, although the findings may not be consistent between species and studies in terms of directional biases in hand use, it is important to emphasise that the TUBE task is a reliable and sensitive measure of hand preference in captive nonhuman primates. The reason for differences between the rhesus and capuchin monkey findings is unclear but could easily arise from rearing or age differences (see Westergaard et al., 1997 for discussion).
Figure 4.
Mean handedness index scores for six species representing eight different studies. Chimp (1) data are from the YNPRC (N = 202). Chimp (2) data were collected at the University of Texas Cancer Center in Bastrop, Texas (N = 116). Gorilla data were collected at Zoo Atlanta and Lincoln Park Zoo (N = 31). Orangutan data were collected at the YNPRC and Zoo Atlanta (N = 19). Rhesus (1) data are from Westergaard & Suomi (1996) (N = 55) and Rhesus (2) data are from Westergaard et al. (1997) (N = 18). Baboon data are from Damerose (unpublished doctoral dissertation) (N = 48). Cebus (1) data are from Spinozzi et al. (1998) (N = 26). Cebus (2) data are from Westergaard & Suomi (1996) (N = 24).
If one considers other measures of hand use in great apes that were not conducted by Hopkins and colleagues, population-level right-handedness has been found in gorillas (Heestand, 1986; Olson, Ellis, & Nadler, 1990; Shafer, 1993), chimpanzees (Colell, Segarra, & Sabater-Pi, 1995; Heestand, 1986), orangutans (Olson et al., 1990), and captive and wild bonobos (Ingmanson, 1996, 1998; Shafer, 1993). Population-level left-handedness has been found for self-directed touching in orangutans (Rogers & Kaplan, 1996) and spontaneous hand use for everyday activities in chimpanzees (Toback, 1999). Thus, in studies with reasonable sample sizes and reliable and sensitive measures of hand use, population-level handedness is evident in other laboratories and settings.
ARE THERE DIFFERENCES IN HAND PREFERENCE DISTRIBUTIONS BETWEEN CAPTIVE AND WILD GREAT APES?
In terms of the biological and evolutionary significance of handedness studies in great apes (and other primates), the issue of whether the effects are restricted to captive populations is an important one. Palmer (2002) has argued that the evidence that does exist for population-level right-handedness in chimpanzees is largely restricted to captive populations and therefore may be an artefact of captivity with little external validity. McGrew and Marchant (1997) have made similar arguments because they have failed to find population-level handedness in two studies of spontaneous hand use in wild chimpanzees. The evidence used by Palmer (2002) and McGrew and Marchant (1997) to make their arguments is weak, and Hopkins has argued elsewhere (1999) that there are as many differences in the types of measures used to assess hand preference between captive and field studies as there are contextual factors that may explain differences in the results between settings. Several examples illustrate this point.
One potential explanation for differences between field and captive studies may be in the types of measures employed between settings. Focusing on studies in chimpanzees, Marchant and McGrew (1996) and McGrew and Marchant (2001) have overwhelmingly focused on spontaneous motor behaviours expressed by the wild chimpanzees in their everyday activities. Such activities include scratch, pull, groom, eat, pick-up, etc. Similarly, Boesch (1991), in addition to tool-use for wadge dipping and stone hammering, examined hand preferences for reaching and grooming. Shown in Table 1 are the hand preference distributions from captive and wild chimpanzees for five behaviours that have been assessed in captive and wild settings, including scratch, groom, eat, pick-up, and hold. Two points are worth noting. First, with the exception of the hold behaviour, the patterns of hand preference are relatively comparable between captive and field settings and do not point to large differences between settings. Second, there are many ambiguously handed subjects in both captive and wild chimpanzees for all of these measures. Much like the bimanual feeding task used by Hopkins (1994), the primary problem with these measures is that they do not appear to be sensitive enough to detect strong individual hand preferences. In short, if one wants to determine whether species exhibit population-level handedness, then one needs a measure that is sensitive enough to reliably detect a consistent hand preference at the individual level. In none of these field studies in chimpanzees do the measures of hand use meet this criterion. Even in humans, measures of hand use or hand activity for spontaneous activities fail to reveal population-level hand preference (Eaton, Rothman, McKeen, & Campbell, 1998; Marchant, McGrew, & Eibs-Eibesfeldt, 1995). Taken together, the validity of these measures of hand use in wild chimpanzees is questionable and most assuredly cannot be compared with the structured measures of hand use often used with captive chimpanzees.
In contrast to measures of spontaneous hand use, measures of tool use appear to detect strong hand preferences at the individual level (Boesch, 1991; Hopkins, Bard, Jones, & Bales, 1993; Hopkins & Rabinowitz, 1997; Marchant, 1983; Matsuzawa, 1994; McGrew & Marchant, 1992; McGrew, Marchant, Wrangham, & Klein, 1999; Sugiyama, Fushimi, Sakura, & Matsuzawa, 1993). Shown in Table 2 are the data on hand preference in wild and captive chimpanzees. McGrew and Marchant (1997) have argued that there is no evidence of population-level handedness in tool use but there are two important issues to consider in this interpretation. One issue has to do with the relative proportion of right- to left-handedness in the entire sample and across samples. With the exception of termite fishing, the general pattern is more right- than left-handed subjects in the wild chimpanzees. This difference in the number of left- and right-handed apes is not statistically significant, but the ratio of right- to left-handed subjects is about 2:1 which is largely consistent with the ratio of right- to left-handed subjects reported in captive chimpanzees (see Hopkins & Pearson, 2000). The reason the 2:1 ratio is significant in captive subjects is because the sample size is usually twice and as many as three times larger than in field studies. In other words, studies in the captive YNPRC chimpanzees have substantially greater statistical power than any single or the combined field studies on hand use in wild chimpanzees or captive chimpanzees. This highlights the need for more studies in wild populations of chimpanzees with a focus on larger sample sizes.
TABLE 2.
Hand preferences for tool use in chimpanzees
| Author(s) | #L | #A | #R | Task |
|---|---|---|---|---|
| Boesch (1991) | 4 | 3 | 9 | Wadge dipping |
| McGrew et al. (1999) | 5 | 2 | 7 | Anvil use |
| Boesch (1991) | 26 | 5 | 34 | Nut cracking |
| Sugiyama et al. (1993)* | 7 | 1 | 12 | Nut cracking |
| Sugiyama (1995) | 2 | 2 | 4 | Wand manipulation |
| McGrew & Marchant (1992)** | 9 | 0 | 6 | Termite fishing |
| Hopkins & Rabinowitz (1997) | 11 | 9 | 16 | Honey dipping |
| Hopkins & Rabinowitz (1997) | 6 | 4 | 18 | Honey dipping |
| Marchant (1983) | 4 | 9 | 5 | Throwing |
| Hopkins et al. (1993) | 9 | 6 | 21 | Throwing |
L = Left, A = Ambiguous, R = Right.
These data were supplemented with data presented by Matsuzawa (1994) and Matsuzawa et al. (2001).
Nishida and Hiraiwa (1982) presented data on termite fishing but there were too few observations to determine individual hand preferences.
A second important issue in comparing field and captive studies has to do with the precision of measurement in assessing hand preference. Numerous studies have demonstrated that situational and postural factors can influence hand preferences in nonhuman primates (see Hopkins & Fernandez-Carriba, 2000; Lehman, 1993; Ward & Cantalupo, 1997). In addition, recent studies suggest that grip morphology has an influence on preferential use of the right and left hand in chimpanzees and other great apes (Christel, 1994; Tonooka & Matsuzawa, 1995). In terms of the potential influence of situational factors, the comparison of the hand preference data for termite fishing by McGrew and Marchant (1992) with the findings on honey dipping in captive primates is of significance. McGrew and Marchant (1992) made no attempt to characterise hand use for termite fishing when using a unimanual contrasted with a bimanual strategy. Hopkins and Rabinowitz (1997), on the other hand, directly compared hand preferences for unimanual contrasted with bimanual strategies for honey dipping, a task designed to simulate termite fishing in wild chimpanzees. Population-level right-handedness was found only for bimanual strategies and not for unimanual strategies. In fact, the findings for the unimanual strategy were similar to those reported for wild chimpanzees. Thus, the differences between captive and wild chimpanzees may reflect more the motor demands of the task than the environment per se (see Table 2).
This point is also highly relevant to the findings by Boesch (1991) and Sugiyama et al. (1993) for nut cracking in wild chimpanzees. Boesch (1991) described the nut-cracking task as a “complex bimanual task” (p. 545) but made no attempt to distinguish between unimanual and bimanual strategies in hand use. In fact, Boesch (1991) argues that the reason the younger animals were more right-handed than the older subjects was because they used exclusively a unimanual strategy. These comments are not intended to berate or criticise the efforts made by field workers but rather to articulate procedural and methodological differences that could just as easily account for the disparate findings between captive and field studies, as the settings themselves. Indeed, we believe that the findings on hand preference for tool use are very interesting and remarkable, given the limited procedural criteria used to evaluate hand preference. That stated, we hope that greater emphasis is placed on the potential influence of situational and positional factors on hand use in future studies in wild great apes.
ARE THERE DIFFERENCES IN HAND PREFERENCE DISTRIBUTIONS BETWEEN WILD CHIMPANZEES?
McGrew and Marchant (1997) have been particularly adamant of their claims of significant differences in hand preference distributions between wild and captive studies. An equally important question is the degree of variation between findings in wild apes. A comparison in findings in hand use between the wild chimpanzees of Mahale, Gombe, and Tai forest reveals that there is substantial variation between sites (see Table 3). The most common measures of assessment come from Mahale and Gombe, both of which were conducted by McGrew and Marchant. What is particularly striking in the findings between sites is the inconsistency in results. Almost none of the common measures of hand use in the Mahale group is sensitive enough to detect significant individual hand preferences, while the very same measures do detect significant preferences in the Gombe group. Why this is the case is not clear because no individual data were presented. If there are fewer observations in the subjects at Mahale compared to Gombe then this might explain the variation.
TABLE 3.
Distribution of hand preferences for 10 measures of hand use in chimpanzees from three field sites
|
Study Site |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Gombe |
Mahale |
Tai |
|||||||
| #L | #R | #A | #L | #R | #A | #L | #R | #A | |
| Scratch | 13 | 23 | 2 | 1 | 0 | 42 | |||
| Groom | 14 | 18 | 2 | 2 | 5 | 29 | 0 | 6 | 9 |
| Eat | 21 | 13 | 4 | 0 | 1 | 40 | |||
| Pluck | 15 | 19 | 2 | 1 | 0 | 39 | |||
| Pick-up | 13 | 8 | 4 | 0 | 0 | 10 | 5 | 6 | 9 |
| Hold | 11 | 13 | 1 | 8 | 12 | 10 | |||
| Nose wipe | 10 | 17 | 5 | 6 | 0 | 32 | |||
| Pull | 22 | 11 | 2 | 0 | 0 | 35 | |||
| Cradle | 3 | 7 | 2 | 1 | 0 | 6 | |||
| Reach | — | — | — | 0 | 0 | 5 | |||
L = Left, A = Ambiguous, R = Right.
Data from Boesch, 1991; Marchant & McGrew, 1996; McGrew & Marchant, 2001; Sugiyama et al., 1993.
The only potential measure that can be compared between all sites is grooming, and the results are very illuminating. When the frequencies of left, right, and ambidextrous chimpanzees are calculated, there are 16, 29, and 40, respectively. A comparison between the number of left- and right-handed subjects reaches statistical significance (z = 3.21, p < .01). In fact, Boesch (1991) reported individual data for all of his measures of hand use in wild chimpanzees and used nonparametric statistics to evaluate population-level handedness. We have taken these raw data and re-analysed them using one-sample t-tests and the results indicate population-level right-handedness for grooming, t(14) = 2.46, p < .05, and borderline population-level right-handedness for wadge dipping, t(16) = 1.55, p < .10. No individual data were presented in the McGrew and Marchant (2001) and the Marchant and McGrew (1996) papers.
In sum, there is as much, if not more, variation in findings between wild chimpanzees from different field sites than there is between any captive and field study. Notwithstanding some measures, population-level right-handedness is apparent in wild chimpanzees. Moreover, nonparametric statistics should not be the sole means of testing for population-level handedness, but rather greater consideration of the attributes of the data sample should guide decisions regarding the types of analyses to employ.
STATISTICAL POWER AND EFFECT SIZE
Lastly, as Hopkins and Pearson (2000) have argued, the magnitude of hand preference is smaller in apes compared to humans and this presents a formidable statistical problem when comparing studies. Specifically, if it is assumed that a true ratio of right- to left-handedness in apes is roughly 2:1 then large sample sizes are needed to detect significant population-level preferences. For example, adopting a beta value of .80 and having six levels of an independent variable(s), such rearing (mother-reared, human-reared wild-caught) and sex (male, female), the sample sizes needed to detect significance at .10, .20, and .40 effect sizes (small, moderate, and large effects) are 138, 66, and 36 respectively. Few, if any, studies on hand preference in great apes have met this criterion (particularly for the small and moderate effect sizes) even though the bulk of the studies point to directional biases in hand use (see Table 4 in Hopkins & Pearson, 2000). This is particularly problematic for measures that are not sensitive enough to detect strong preferences in each individual subject. As stated previously, this is a fundamental difference between the YNPRC studies and nearly all other studies on hand preference in great apes and should not be overlooked just because the animals are in captivity. A further statistical artefact in the existing literature is the nearly exclusive use of chi-square analysis rather than one-sample t-tests. Investigators opt for use of chi-square tests because they usually classify subjects into categories of hand preference based on z-scores. This is not the only or arguably the best way to analyse hand preference data and others have argued that the application of parametric rather than nonparametric statistics to hand preference data is a reasonable and more powerful approach in evaluating for population-level hand preference (see Hopkins, 1999).
SUMMARY
In summary, the application of funnel graphs to hand preference seems appropriate when there are unequal sample sizes in the number of observations used to evaluate individual hand preferences. Although the application of funnel graphs by Palmer (2002) to the Hopkins (1994) data did illuminate some apparent anomalies in the data, we do not believe it entirely explains the results of that study and, in particular, other studies of hand preference in chimpanzees. Moreover, the application of funnel graphs to other handedness data sets from the chimpanzees at the YNPRC does not account for the reported population-level right-handedness in these chimpanzees.
The generality of the handedness findings from the YNPRC to wild chimpanzees and chimpanzees in other captive settings remains unclear. The studies conducted by Hopkins and colleagues in the YNPRC colony differ from other studies in three important ways including (a) sample size, (b) type(s) of measure, and (c) experimental control over situational factors. The studies in the YNPRC chimpanzees have typically involved structured rather than unstructured measures of hand use and relatively large sample sizes, and efforts have been made to control or account for potential extraneous variables such as manual strategy (Hopkins & Rabinowitz, 1997), posture (Hopkins, 1993; Hopkins et al., 1993) or situational or positional factors (Hopkins et al., 2001). All of these factors alone or in conjunction can just as easily explain differences in chimpanzee hand preference between settings as the purported explanations of environmental or social learning mechanisms. Until such time, we believe it is better to accumulate a larger corpus of data and focus on collecting comparable data from field and captive settings, rather than highlight differences in findings, when confronted with the apple and oranges problem. To this end, our understanding of the evolution of handedness in humans and apes will be enhanced.
Acknowledgments
This work was supported in part by NIH grants RR-00165, NS-29574, NS-36605, NS-42867 and HD-38051.
Contributor Information
William D. Hopkins, Berry College, Mount Berry, GA, and Yerkes National Primate Research Center, Atlanta, GA, USA
Claudio Cantalupo, Yerkes National Primate Research Center and Georgia State University, Atlanta, GA, USA.
REFERENCES
- Boesch C. Handedness in wild chimpanzees. International Journal of Primatology. 1991;6:541–558. [Google Scholar]
- Bradshaw J, Rogers LJ. The evolution of lateral asymmetries, language, tool use and intellect. Academic Press, Inc.; San Diego: 1993. [Google Scholar]
- Christel MI. Catarrhine primates grasping small objects: Techniques and hand preferences. In: Anderson JR, Roeder JJ, Thierry B, Herrenschmidt N, editors. Current primatology. Vol. IV: Behavioral neuroscience, physiology and reproduction. Université Louis Pasteur; Strasbourg: 1994. pp. 37–49. [Google Scholar]
- Colell M, Segarra MD, Sabater-Pi J. Hand preferences in chimpanzees (Pan troglodytes), bonobos (Pan paniuscus) and orangutans (Pongo pygmaeus) in food-reaching and other daily activities. International Journal of Primatology. 1995;16:413–434. [Google Scholar]
- Eaton WO, Rothman DB, McKeen NA, Campbell DW. Something sinistral going on? Asymmetry in arm movement frequency. Laterality. 1998;3:311–322. doi: 10.1080/713754318. [DOI] [PubMed] [Google Scholar]
- Fagot J, Vauclair J. Manual laterality in nonhuman primates: A distinction between handedness and manual specialization. Psychological Bulletin. 1991;109:76–89. doi: 10.1037/0033-2909.109.1.76. [DOI] [PubMed] [Google Scholar]
- Heestand J. Behavioral lateralization in four species of ape. University of Washington; Seattle: 1986. Unpublished doctoral dissertation. [Google Scholar]
- Hook-Costigan MA, Rogers LJ. Hand preferences in New World primates. International Journal of Comparative Psychology. 1997;9:173–207. [Google Scholar]
- Hopkins WD. Posture and reaching in chimpanzees (Pan) and orangutans (Pongo) Journal of Comparative Psychology. 1993;17:162–168. doi: 10.1037/0735-7036.107.2.162. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for bimanual feeding in 140 captive chimpanzees (Pan troglodytes): Rearing and ontogenetic factors. Developmental Psychobiology. 1994;27:395–407. doi: 10.1002/dev.420270607. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees (Pan troglodytes): Cross-sectional analysis. Journal of Comparative Psychology. 1995;105:178–190. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Chimpanzee handedness revisited: 55 years since Finch. Psychonomic Bulletin and Review. 1996;1941;3:449–457. doi: 10.3758/BF03214548. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. On the other hand: Statistical issues in the assessment and interpretation of hand preference data in nonhuman primates. International Journal of Primatology. 1999;20:851–866. [Google Scholar]
- Hopkins WD, Bard KA, Jones A, Bales S. Chimpanzee hand preference for throwing and infant cradling: Implications for the origin of human handedness. Current Anthropology. 1993;34:786–790. [Google Scholar]
- Hopkins WD, Cantalupo C. Does variation in sample size explain individual differences in hand preferences of chimpanzees: A rely to Palmer. American Journal of Physical Anthropology. 2003;2002;121:878–881. doi: 10.1002/ajpa.10170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Fernanadez-Carriba S. The effect of situational factors on hand preference in chimpanzees (Pan troglodytes) Neuropsychologia. 2000;38:403–409. doi: 10.1016/s0028-3932(99)00092-5. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Fernanadez-Carriba S, Wesley MJ, Hostetter A, Pilcher D, Poss S. The use of bouts and frequencies in the evaluation of hand preferences for a coordinated bimanual task in chimpanzees (Pan troglodytes): An empirical study comparing two different indices of laterality. Journal of Comparative Psychology. 2001;115:294–299. doi: 10.1037//0735-7036.115.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Hook M, Braccini S, Schapiro S. Population-level right handedness for a coordinated bimanual task in chimpanzees (Pan troglodytes): Replication and extension in a second colony of apes. International Journal of Primatology. 2003a;24:677–689. doi: 10.1023/A:1023752816951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Morris RD. Handedness in great apes. A review of findings. International Journal of Primatology. 1993;14:1–25. [Google Scholar]
- Hopkins WD, Pearson K. Chimpanzee (Pan troglodytes) handedness: Variability across multiple measures of hand use. Journal of Comparative Psychology. 2000;114:126–135. doi: 10.1037/0735-7036.114.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Rabinowitz DM. Manual specialization and tool-use in captive chimpanzees (Pan troglodytes): The effect of unimanual and bimanual strategies on hand preference. Laterality. 1997;2:267–277. doi: 10.1080/713754273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Stoinski T, Lukas KE, Ross SR, Wesley MJ. Comparative assessment of handedness for a coordinated bimanual task in chimpanzees (Pan), gorillas (Gorilla), and orangutans (Pongo) Journal of Comparative Psychology. 2003b;117:302–308. doi: 10.1037/0735-7036.117.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Wesley MJ, Izard K, Hook M, Schapiro SJ. Chimpanzees are right-handed: Replication in three populations of apes. Behavioral Neuroscience. doi: 10.1037/0735-7044.118.3.659. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingmanson EJ. Hand use preference among Pan paniscus at Wamba, Zaire; Paper presented at the annual meeting of the American Association of Physical Anthropologists; Raleigh-Durham, North Carolina. 1996. [Google Scholar]
- Ingmanson EJ. Right and left hand use preference in a power grip task among Pan paniscus at Wamba; Paper presented at the annual meeting of the American Association of Physical Anthropologists; Salt Lake City, Utah. 1998. [Google Scholar]
- Leavens DA, Aureli F, Hopkins WD, Hyatt CW. Effect of cognitive challenge on self-directed behaviors by chimpanzees (Pan troglodytes) American Journal of Primatology. 2001;55:1–14. doi: 10.1002/ajp.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman RAW. Manual preference in prosimians, monkeys, and apes. In: Ward JP, Hopkins WD, editors. Primate laterality: Current behavioral evidence of primate asymmetries. Springer-Verlag; New York: 1993. pp. 107–124. [Google Scholar]
- Marchant LF. Hand preferences among captive island groups of chimpanzees. Rutgers, The State University of New Jersey; New Brunswick, USA: 1983. Unpublished doctoral dissertation. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of function in apes: A meta-analysis of methods. Journal of Human Evolution. 1991;21:425–438. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of limb function in wild chimpanzees of Gombe National Park: Comprehensive study of spontaneous activities. Journal of Human Evolution. 1996;30:427–443. [Google Scholar]
- Marchant LF, McGrew WC, Eibs-Eibesfeldt I. Is human handedness universal? Ethological analysis from three different cultures. Ethology. 1995;101:239–258. [Google Scholar]
- Matsuzawa T. Field experiments on the use of stone tools by chimpanzees in the wild. In: Wrangham RW, McGrew WC, de Waal FBM, Heltne PG, editors. Chimpanzee cultures. Cambridge University Press; Cambridge, MA: 1994. pp. 351–370. [Google Scholar]
- Matsuzawa T, Biro D, Humle T, Inoue-Nakamura N, Tonooka R, Yamakoshi G. Emergence of culture in wild chimpanzees: Education by master-apprenticeship. In: Matsuzawa T, editor. Primate origins of human cognition and behavior. Springer; Tokyo: 2001. pp. 557–574. [Google Scholar]
- McGrew WC, Marchant LF. Chimpanzees, tools, and termites: Hand preference or handedness? Current Anthropology. 1992;33:114–119. [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: Current issues in and meta-analysis of the behavioral laterality of hand function in nonhuman primates. Yearbook of Physical Anthropology. 1997;40:201–232. [Google Scholar]
- McGrew WC, Marchant LF. Ethological study of manual laterality in the chimpanzees of the Mahale mountains, Tanzania. Behaviour. 2001;138:329–358. [Google Scholar]
- McGrew WC, Marchant LF, Wrangham RW, Klein H. Manual laterality in anvil use: Wild chimpanzees cracking Strychnos fruits. Laterality. 1999;4:79–87. doi: 10.1080/03069887600760101. [DOI] [PubMed] [Google Scholar]
- Nishida T, Hiraiwa M. Natural history of a tool-using behavior by wild chimpanzees in feeding upon wood-boring ants. Journal of Human Evolution. 1982;11:73–99. [Google Scholar]
- Palmer AR. Chimpanzee right-handedness reconsidered: Evaluating the evidence with funnel plots. American Journal of Physical Anthropology. 2002;118:191–199. doi: 10.1002/ajpa.10063. [DOI] [PubMed] [Google Scholar]
- Palmer AR. Reply to Hopkins and Cantalupo: Chimpanzee right-handedness reconsidered: Sampling issues and data presentation. American Journal of Physical Anthropology. 2003;121:382–384. [Google Scholar]
- Rogers LJ, Kaplan G. Hand preferences and other lateral biases in rehabilitated orangutans. Animal Behaviour. 1996;51:13–25. [Google Scholar]
- Shafer DD. Patterns of hand preference in gorillas and children. In: Ward JP, Hopkins WD, editors. Primate laterality: Current behavioral evidence of primate asymmetries. Springer-Verlag; New York: 1993. pp. 267–283. [Google Scholar]
- Spinozzi G, Castornina MG, Truppa V. Hand preferences in unimanual and coordinated-bimanual tasks by tufted capuchin monkeys (Cebus apella) Journal of Comparative Psychology. 1998;112:183–191. [Google Scholar]
- Steiner SM. Handedness in chimpanzees. Friends of Washoe. 1990;9:9–19. [Google Scholar]
- Sugiyama Y. Tool-use for catching ants by chimpanzees at Bossou and Monts Nimba, West Africa. Primates. 1995;36:193–205. [Google Scholar]
- Sugiyama Y, Fushimi T, Sakura O, Matsuzawa T. Hand preference and tool use in wild chimpanzees. Primates. 1993;34:151–159. [Google Scholar]
- Toback EL. Laterality of manual and pedal activity in captive chimpanzees (Pan troglodytes) University of Sterling; UK: 1999. Unpublished doctoral dissertation. [Google Scholar]
- Tonooka R, Matsuzawa T. Hand preferences in captive chimpanzees (Pan troglodytes) in simple reaching for food. International Journal of Primatology. 1995;16:17–34. [Google Scholar]
- Ward JP, Cantalupo C. Origins and functions of laterality: Interactions of motoric systems. In: Fagot J, Rogers L, Ward J, Bulman-Fleming B, Hopkins W, editors. Hemispheric specialisation in animals and humans. Psychology Press; Hove, UK: 1997. pp. 279–303. [Google Scholar]
- Ward JP, Hopkins WD. Primate laterality: Current behavioral evidence of primate asymmetries. Springer-Verlag; New York: 1993. [Google Scholar]
- Westergaard GC, Suomi SJ. Hand preference for a bimanual task in tufted capuchins (Cebus apella) and rhesus macaques (Macaca mulatta) Journal of Comparative Psychology. 1996;110:406–411. doi: 10.1037/0735-7036.110.4.406. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Champoux M, Suomi SJ. Hand preference in infant rhesus macaques (Macaca mulatta) Child Development. 1997;68:387–393. doi: 10.1111/j.1467-8624.1997.tb01946.x. [DOI] [PubMed] [Google Scholar]