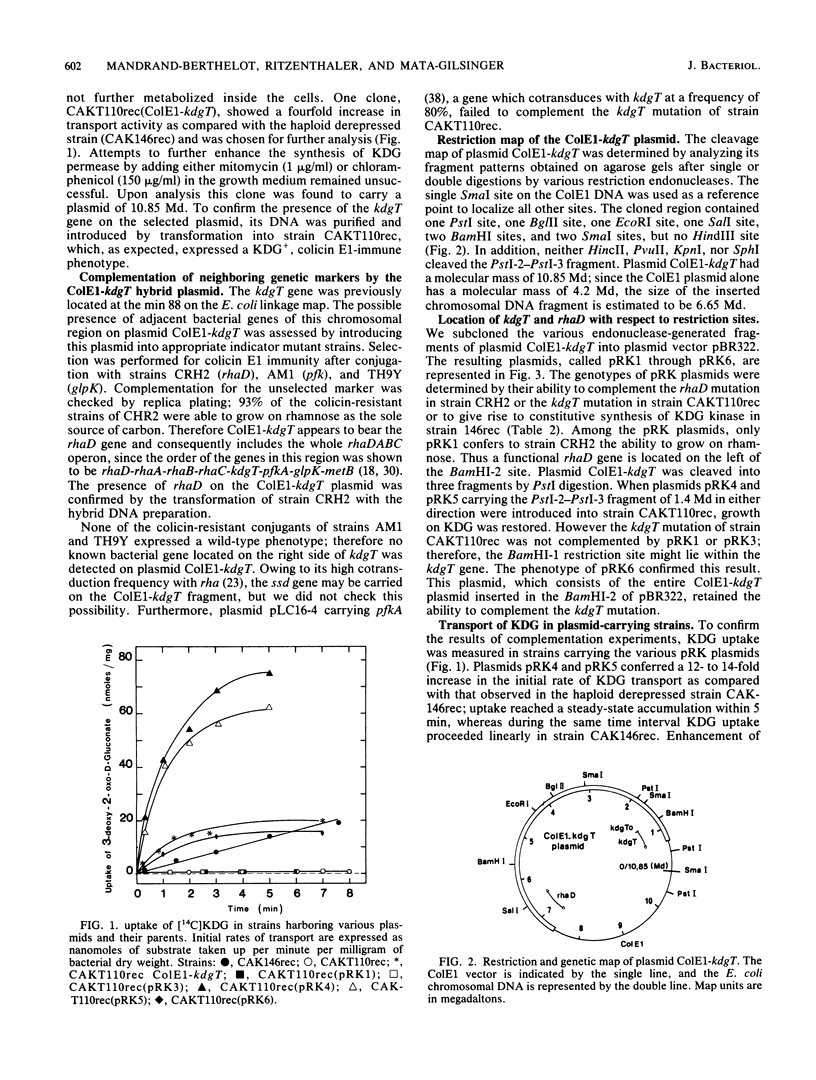
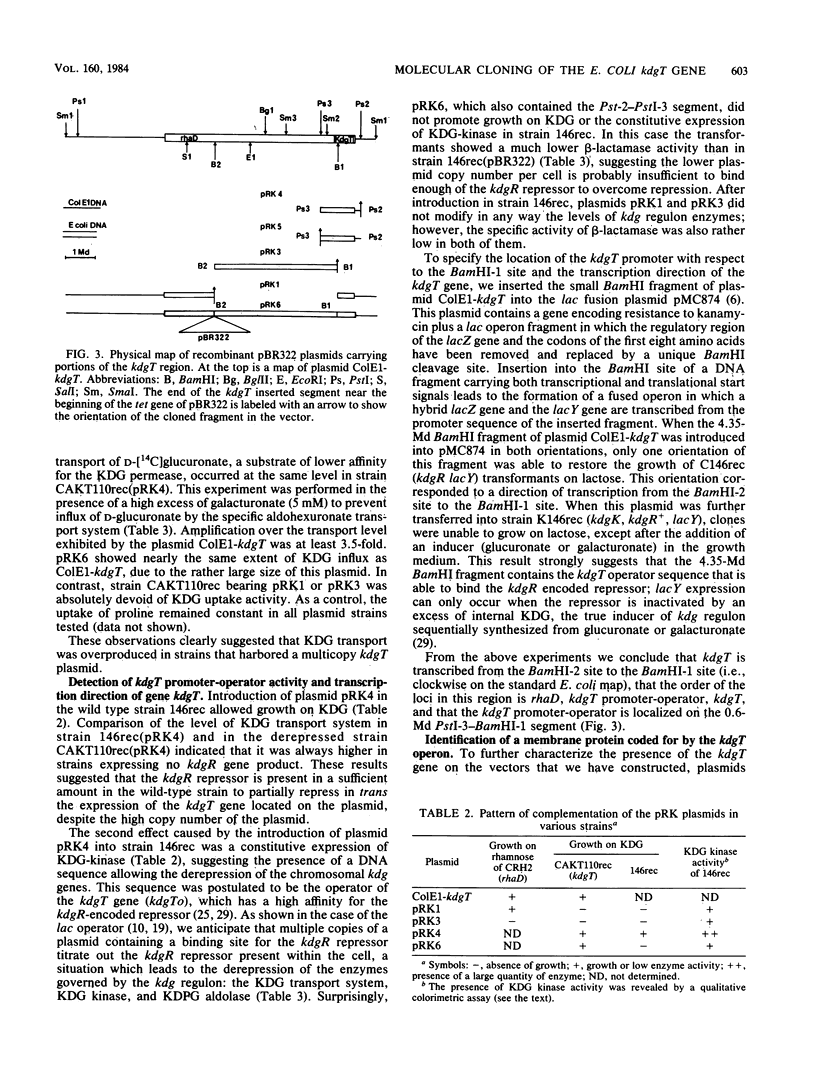
Abstract
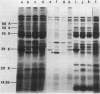
The kdgT gene of Escherichia coli, which encodes for the 3-deoxy-2-oxo-D-gluconate transport system, was isolated as a ColE1-kdgT hybrid plasmid from the Clarke and Carbon bank. A restriction and genetic map of the min 88 region of the chromosome was established; by subcloning the restriction fragments into the plasmid vector pBR322, the kdgT gene was localized on a 1.4-megadalton PstI DNA fragment, and the direction of transcription of the gene was determined by making use of an in vitro gene fusion between kdgT and lacZ genes. Amplification of the gene product of kdgT was up to 14-fold the level found in a haploid strain. When plasmids bearing kdgT were expressed in an in vivo maxicell system, a specific polypeptide of 28,000 daltons appeared that was found to be associated with the membrane fraction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Büchel D. E., Gronenborn B., Müller-Hill B. Sequence of the lactose permease gene. Nature. 1980 Feb 7;283(5747):541–545. doi: 10.1038/283541a0. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Ehring R., Beyreuther K., Wright J. K., Overath P. In vitro and in vivo products of E. coli lactose permease gene are identical. Nature. 1980 Feb 7;283(5747):537–540. doi: 10.1038/283537a0. [DOI] [PubMed] [Google Scholar]
- Heyneker H. L., Shine J., Goodman H. M., Boyer H. W., Rosenberg J., Dickerson R. E., Narang S. A., Itakura K., Lin S., Riggs A. D. Synthetic lac operator DNA is functional in vivo. Nature. 1976 Oct 28;263(5580):748–752. doi: 10.1038/263748a0. [DOI] [PubMed] [Google Scholar]
- Lagarde A. E., Haddock B. A. Proton uptake linked to the 3-deoxy-2-oxo-d-gluconate-transport system of Escherichia coli. Biochem J. 1977 Jan 15;162(1):183–187. doi: 10.1042/bj1620183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde A. E., Pouysségur J. M., Stoeber F. R. A transport system for 2-keto-3-deoxy-D-gluconate uptake in Escherichia coli K12. Biochemical and physiological studies in whole cells. Eur J Biochem. 1973 Jul 16;36(2):328–341. doi: 10.1111/j.1432-1033.1973.tb02917.x. [DOI] [PubMed] [Google Scholar]
- Lagarde A. E., Stoeber F. R. Escherichia coli K-12 structural kdgT mutants exhibiting thermosensitive 2-keto-3-deoxy-D-gluconate uptake. J Bacteriol. 1977 Feb;129(2):606–615. doi: 10.1128/jb.129.2.606-615.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde A. Evidence for an electrogenic 3-deoxy-2-oxo-D-gluconate--proton co-transport driven by the protonmotive force in Escherichia coli K12. Biochem J. 1977 Nov 15;168(2):211–221. doi: 10.1042/bj1680211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson T. J., Schumacher G., Boos W. Identification of the glpT-encoded sn-glycerol-3-phosphate permease of Escherichia coli, an oligomeric integral membrane protein. J Bacteriol. 1982 Dec;152(3):1008–1021. doi: 10.1128/jb.152.3.1008-1021.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson A. J., Jones-Mortimer M. C., Horne P., Henderson P. J. Identification of the GalP galactose transport protein of Escherichia coli. J Biol Chem. 1983 Apr 10;258(7):4390–4396. [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Mandrand-Berthelot M. A., Lagarde A. E. Altered transport properties in Escherichia coli mutants selected for pH-conditional growth on 3-deoxy-2-oxo-D-gluconate. J Biol Chem. 1982 Aug 10;257(15):8806–8816. [PubMed] [Google Scholar]
- Marians K. J., Wu R., Stawinski J., Hozumi T., Narang S. A. Cloned synthetic lac operator DNA is biologically active. Nature. 1976 Oct 28;263(5580):744–748. doi: 10.1038/263744a0. [DOI] [PubMed] [Google Scholar]
- Motojima K., Yamato I., Anraku Y., Nishimura A., Hirota Y. Amplification and characterization of the proline transport carrier of Escherichia coli K-12 by using proT+ hybrid plasmids. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6255–6259. doi: 10.1073/pnas.76.12.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoz G., Robert-Baudouy J., Stoeber F. Physiological and genetic regulation of the aldohexuronate transport system in Escherichia coli. J Bacteriol. 1976 Aug;127(2):706–718. doi: 10.1128/jb.127.2.706-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E. B., Malik N., Walker C. L-serine degradation in Escherichia coli K-12: directly isolated ssd mutants and their intragenic revertants. J Bacteriol. 1982 May;150(2):710–715. doi: 10.1128/jb.150.2.710-715.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyssegur J., Stoeber F. Etude du rameu dégradatif commun des hexuronates chez Escherichia coli K 12. Purification, propriétés et individualité de la 2-céto-3-désoxy-D-gluconokinase. Biochimie. 1971;53(6):771–781. [PubMed] [Google Scholar]
- Pouyssegur J., Stoeber F. Genetic control of the 2-keto-3-deoxy-d-gluconate metabolism in Escherichia coli K-12: kdg regulon. J Bacteriol. 1974 Feb;117(2):641–651. doi: 10.1128/jb.117.2.641-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyssegur J., Stoeber F. Synthèse enzymatique du 2-céto-3-désoxy-D-gluconate. Bull Soc Chim Biol (Paris) 1970;52(12):1419–1428. [PubMed] [Google Scholar]
- Pouysségur J. M., Stoeber F. R. Etude du rameau dégradatif commun des hexuronates chez Escherichia coli K 12. Purification, propriétés et individualité de la 2-céto-3-désoxy-6-phospho-D-gluconate aldolase. Eur J Biochem. 1971 Aug 16;21(3):363–373. doi: 10.1111/j.1432-1033.1971.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Lagarde A. Système de transport du 2-céto-3-désoxy-gluconate chez E. coli K 12: localisation d'un gène de structure et de son opérateur. Mol Gen Genet. 1973 Mar 1;121(2):163–180. doi: 10.1007/BF00277530. [DOI] [PubMed] [Google Scholar]
- Power J. The L-rhamnose genetic system in Escherichia coli K-12. Genetics. 1967 Mar;55(3):557–568. doi: 10.1093/genetics/55.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzenthaler P., Mata-Gilsinger M., Stoeber F. Molecular cloning of Escherichia coli K-12 hexuronate system genes: exu region. J Bacteriol. 1981 Jan;145(1):181–190. doi: 10.1128/jb.145.1.181-190.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R. On the physical state of the intracellularly accumulates substrates of beta-galactoside-permease in Escherichia coli. Biochim Biophys Acta. 1958 Sep;29(3):579–587. doi: 10.1016/0006-3002(58)90015-5. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Horn V., Yanofsky C. On the production of deletions in the chromosome of Escherichia coli. J Mol Biol. 1970 Oct 14;53(1):49–67. doi: 10.1016/0022-2836(70)90045-8. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Nordström K. Microiodometric determination of beta-lactamase activity. Antimicrob Agents Chemother. 1972 Feb;1(2):94–99. doi: 10.1128/aac.1.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Bramhall J., Riede I., Wright J. K., Fürst M., Aichele G., Wilhelm U., Overath P. Lactose carrier protein of Escherichia coli. Structure and expression of plasmids carrying the Y gene of the lac operon. Eur J Biochem. 1980;108(1):223–231. doi: 10.1111/j.1432-1033.1980.tb04715.x. [DOI] [PubMed] [Google Scholar]
- Weiner J. H., Lohmeier E., Schryvers A. Cloning and expression of the glycerol-3-phosphate transport genes of Escherichia coli. Can J Biochem. 1978 Jun;56(6):611–617. doi: 10.1139/o78-092. [DOI] [PubMed] [Google Scholar]
- Yang H. L., Zubay G., Levy S. B. Synthesis of an R plasmid protein associated with tetracycline resistance is negatively regulated. Proc Natl Acad Sci U S A. 1976 May;73(5):1509–1512. doi: 10.1073/pnas.73.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]