Abstract
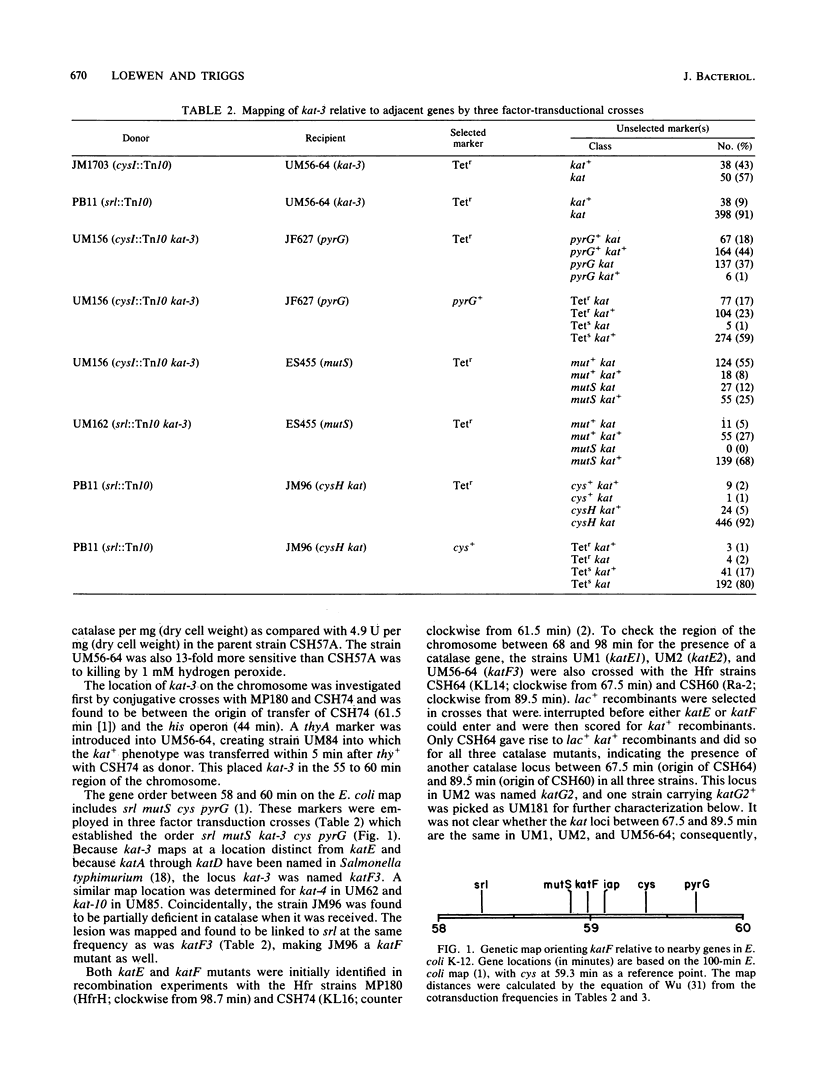
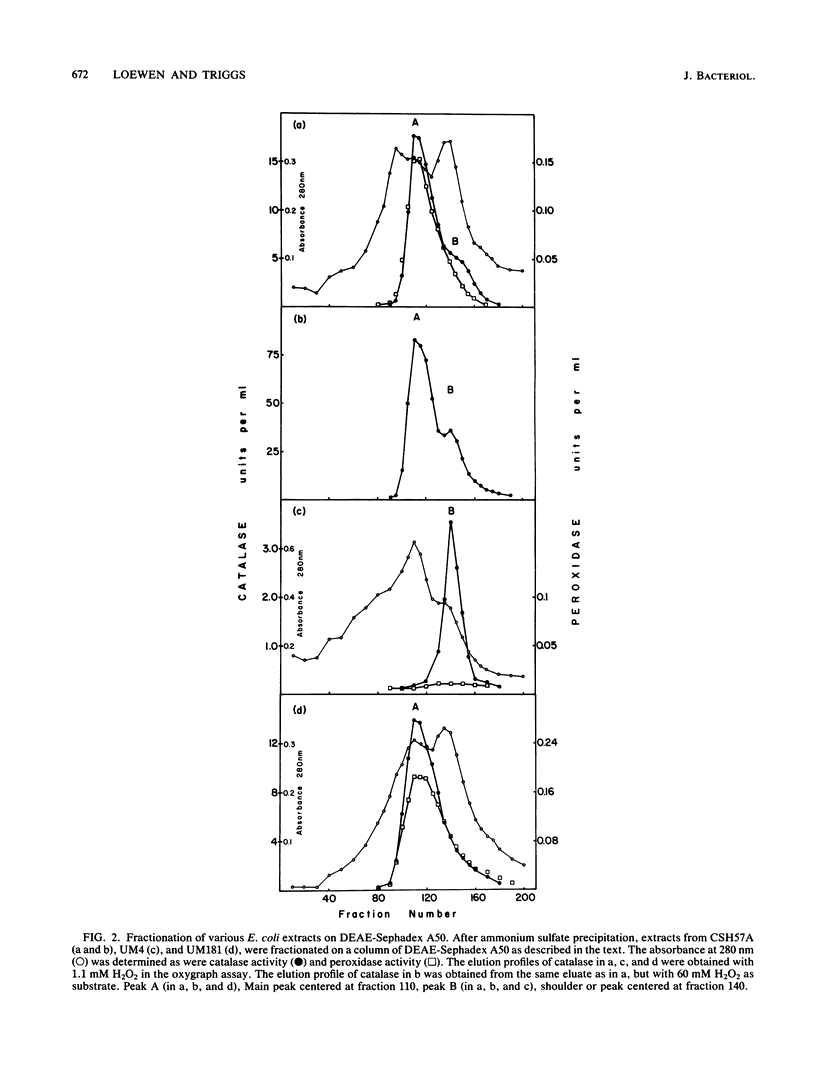
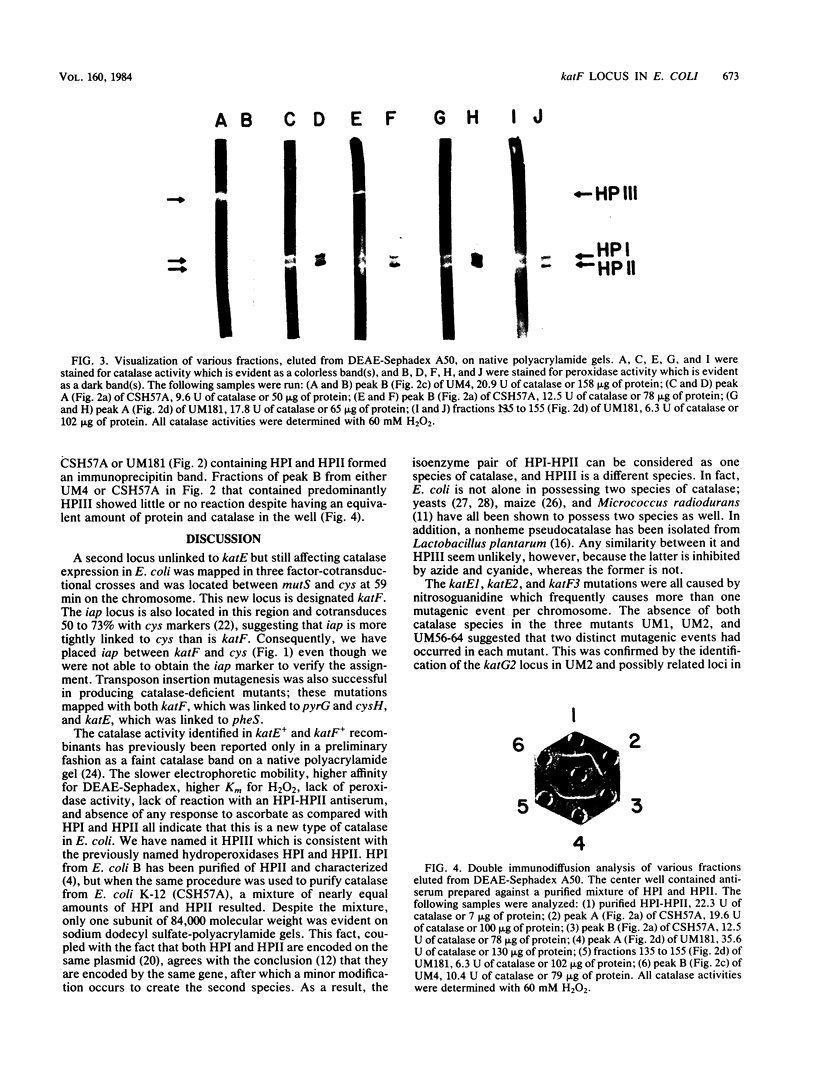
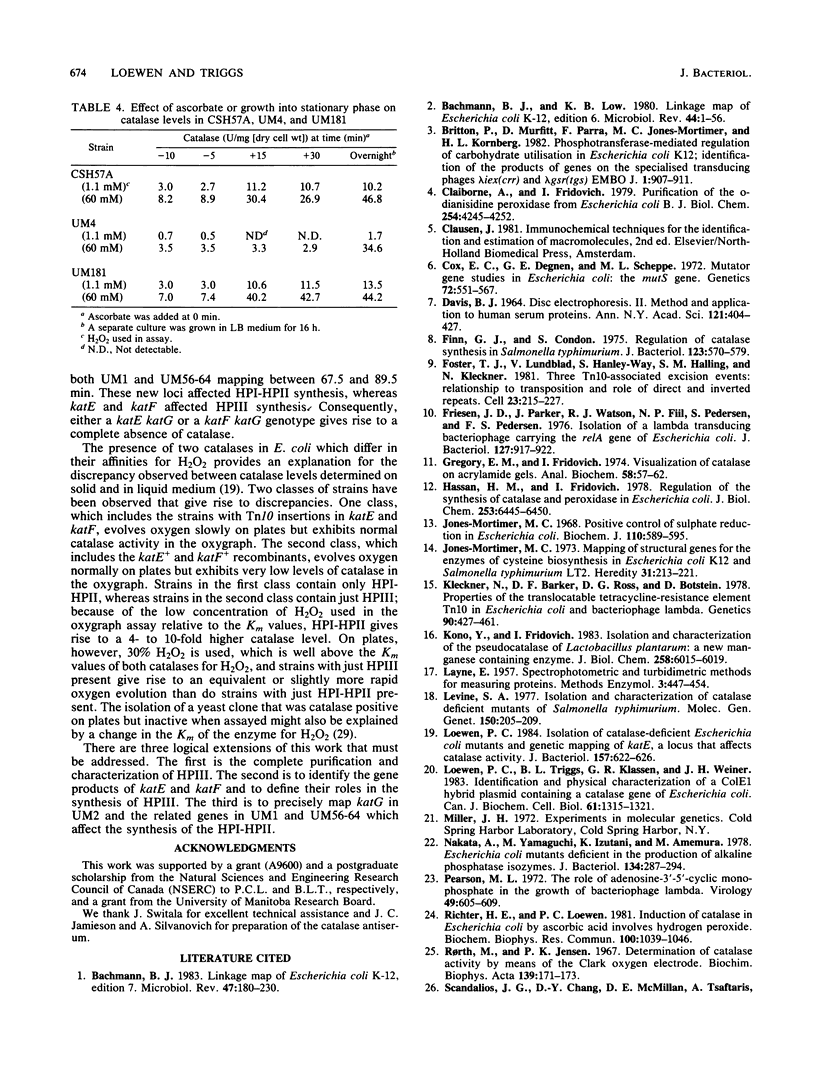
A class of catalase-deficient mutants that was unlinked to katE was localized between mutS and cys at 59.0 min on the Escherichia coli genome. This locus was named katF. Transposon Tn10 insertions were isolated that mapped in both katE and katF loci. The catalase species present in katE+ and katF+ recombinants was found to be different from the main catalase activities, HPI and HPII, in several respects. It did not have an associated peroxidase activity; it was electrophoretically slower on native polyacrylamide gels; it eluted from DEAE-Sephadex A50 at a higher salt concentration; its Km for H2O2 was 30.9 mM as compared with 3.7 mM for HPI and HPII; its synthesis was not induced by ascorbate; and it did not cross react with HPI-HPII antisera. This new catalase was labeled HPIII.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton P., Murfitt D., Parra F., Jones-Mortimer M. C., Kornberg H. L. Phosphotransferase-mediated regulation of carbohydrate utilisation in Escherichia coli K12: identification of the products of genes on the specialised transducing phages lambda iex (crr) and lambda gsr (tgs). EMBO J. 1982;1(8):907–911. doi: 10.1002/j.1460-2075.1982.tb01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claiborne A., Fridovich I. Purification of the o-dianisidine peroxidase from Escherichia coli B. Physicochemical characterization and analysis of its dual catalatic and peroxidatic activities. J Biol Chem. 1979 May 25;254(10):4245–4252. [PubMed] [Google Scholar]
- Cox E. C., Degnen G. E., Scheppe M. L. Mutator gene studies in Escherichia coli: the mutS gene. Genetics. 1972 Dec;72(4):551–567. doi: 10.1093/genetics/72.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Finn G. J., Condon S. Regulation of catalase synthesis in Salmonella typhimurium. J Bacteriol. 1975 Aug;123(2):570–579. doi: 10.1128/jb.123.2.570-579.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J., Lundblad V., Hanley-Way S., Halling S. M., Kleckner N. Three Tn10-associated excision events: relationship to transposition and role of direct and inverted repeats. Cell. 1981 Jan;23(1):215–227. doi: 10.1016/0092-8674(81)90286-5. [DOI] [PubMed] [Google Scholar]
- Friesen J. D., Parker J., Watson R. J., Fill N. P., Pedersen S., Pedersen F. S. Isolation of a lambda transducing bacteriophage carrying the relA gene of Escherichia coli. J Bacteriol. 1976 Aug;127(2):917–922. doi: 10.1128/jb.127.2.917-922.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Visualization of catalase on acrylamide gels. Anal Biochem. 1974 Mar;58(1):57–62. doi: 10.1016/0003-2697(74)90440-0. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Regulation of the synthesis of catalase and peroxidase in Escherichia coli. J Biol Chem. 1978 Sep 25;253(18):6445–6420. [PubMed] [Google Scholar]
- Jones-Mortimer M. C. Mapping of structural genes for the enzymes of cysteine biosynthesis in Escherichia coli K12 and Salmonella typhimurium LT2. Heredity (Edinb) 1973 Oct;31(2):213–221. doi: 10.1038/hdy.1973.76. [DOI] [PubMed] [Google Scholar]
- Jones-Mortimer M. C. Positive control of sulphate reduction in Escherichia coli. Isolation, characterization and mapping oc cysteineless mutants of E. coli K12. Biochem J. 1968 Dec;110(3):589–595. doi: 10.1042/bj1100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Barker D. F., Ross D. G., Botstein D. Properties of the translocatable tetracycline-resistance element Tn10 in Escherichia coli and bacteriophage lambda. Genetics. 1978 Nov;90(3):427–461. doi: 10.1093/genetics/90.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono Y., Fridovich I. Isolation and characterization of the pseudocatalase of Lactobacillus plantarum. J Biol Chem. 1983 May 25;258(10):6015–6019. [PubMed] [Google Scholar]
- Levine S. A. Isolation and characterization of catalase deficient mutants of Salmonella typhimurium. Mol Gen Genet. 1977 Jan 18;150(2):205–209. doi: 10.1007/BF00695400. [DOI] [PubMed] [Google Scholar]
- Loewen P. C. Isolation of catalase-deficient Escherichia coli mutants and genetic mapping of katE, a locus that affects catalase activity. J Bacteriol. 1984 Feb;157(2):622–626. doi: 10.1128/jb.157.2.622-626.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen P. C., Triggs B. L., Klassen G. R., Weiner J. H. Identification and physical characterization of a Col E1 hybrid plasmid containing a catalase gene of Escherichia coli. Can J Biochem Cell Biol. 1983 Dec;61(12):1315–1321. doi: 10.1139/o83-168. [DOI] [PubMed] [Google Scholar]
- Nakata A., Yamaguchi M., Izutani K., Amemura M. Escherichia coli mutants deficient in the production of alkaline phosphatase isozymes. J Bacteriol. 1978 Apr;134(1):287–294. doi: 10.1128/jb.134.1.287-294.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson M. L. The role of adenosine 3',5'-cyclic monophosphate in the growth of bacteriophage lambda. Virology. 1972 Aug;49(2):605–609. doi: 10.1016/0042-6822(72)90513-2. [DOI] [PubMed] [Google Scholar]
- Richter H. E., Loewen P. C. Induction of catalase in Escherichia coli by ascorbic acid involves hydrogen peroxide. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1039–1046. doi: 10.1016/0006-291x(81)91928-8. [DOI] [PubMed] [Google Scholar]
- Rorth M., Jensen P. K. Determination of catalase activity by means of the Clark oxygen electrode. Biochim Biophys Acta. 1967 May 16;139(1):171–173. doi: 10.1016/0005-2744(67)90124-6. [DOI] [PubMed] [Google Scholar]
- Scandalios J. G., Chang D. Y., McMillin D. E., Tsaftaris A., Moll R. H. Genetic regulation of the catalase developmental program in maize scutellum: Identification of a temporal regulatory gene. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5360–5364. doi: 10.1073/pnas.77.9.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seah T. C., Bhatti A. R., Kaplan J. G. Novel catalatic proteins of bakers' yeast. I. An atypical catalase. Can J Biochem. 1973 Nov;51(11):1551–1555. doi: 10.1139/o73-208. [DOI] [PubMed] [Google Scholar]
- Seah T. C., Kaplan J. G. Purification and properties of the catalase of bakers' yeast. J Biol Chem. 1973 Apr 25;248(8):2889–2893. [PubMed] [Google Scholar]
- Spevak W., Fessl F., Rytka J., Traczyk A., Skoneczny M., Ruis H. Isolation of the catalase T structural gene of Saccharomyces cerevisiae by functional complementation. Mol Cell Biol. 1983 Sep;3(9):1545–1551. doi: 10.1128/mcb.3.9.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]





