Abstract
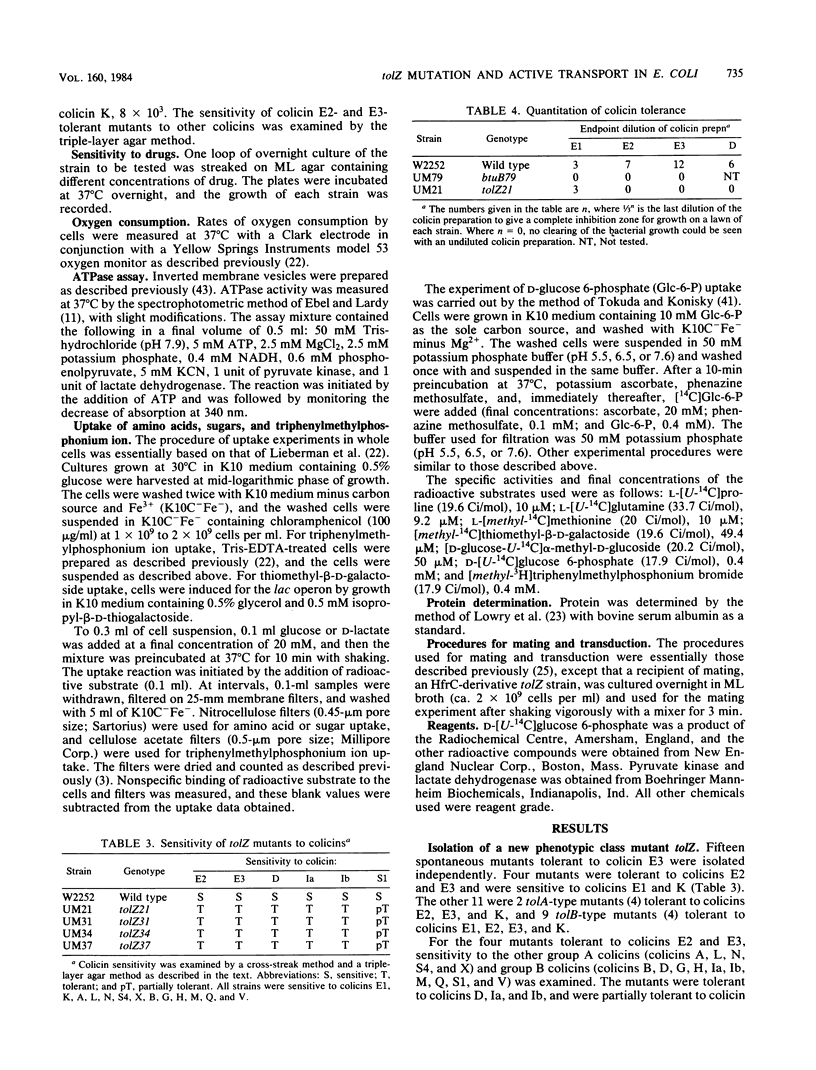
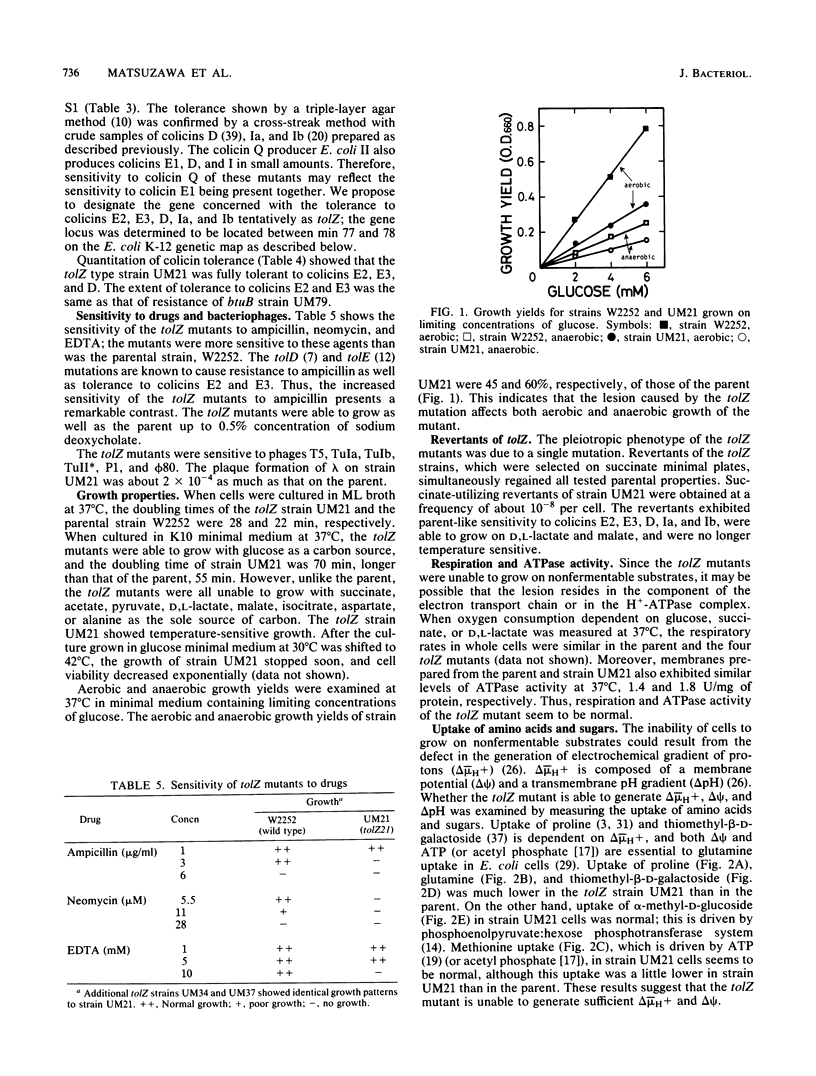
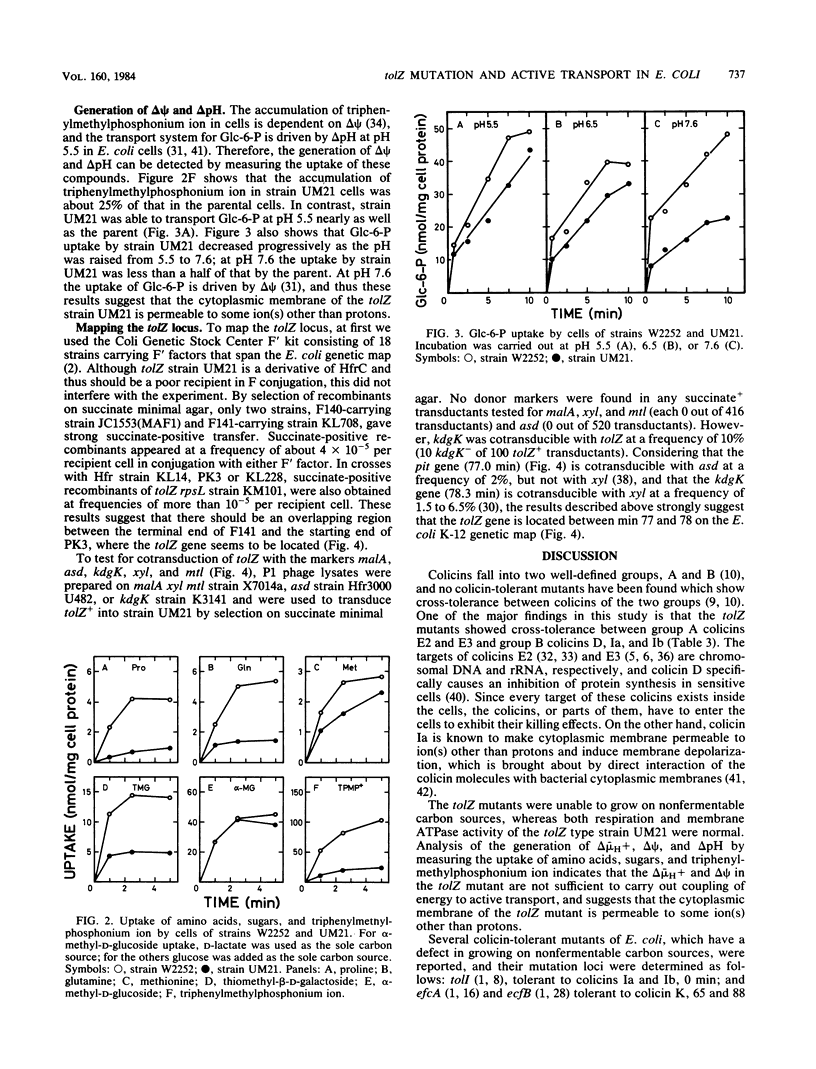
Spontaneous Escherichia coli K-12 mutants tolerant to colicin E3 were isolated, and on the basis of their tolerance patterns to 19 kinds of colicins, a new phenotypic class of tolZ mutants was found. The tolZ gene was located between min 77 and 78 on the E. coli K-12 genetic map. The tolZ mutants were tolerant to colicins E2, E3, D, Ia, and Ib, and showed an increased sensitivity to ampicillin, neomycin, and EDTA, but not to deoxycholate; they were able to grow on glucose minimal medium, but not on nonfermentable carbon sources (succinate, acetate, pyruvate, lactate, malate, etc.). The pleiotropic phenotype of the tolZ mutant was due to a single mutation. Both respiration and membrane ATPase activity of the tolZ mutant were normal. The tolZ mutant had a defect in the uptake of proline, glutamine, thiomethyl-beta-D-galactoside, and triphenylmethylphosphonium ion; these uptake systems are driven by an electrochemical proton gradient (delta-mu H+) or a membrane potential (delta psi). In contrast, the uptake of methionine and alpha-methyl-D-glucoside, which is not dependent on delta-mu H+ and delta psi, was normal in the tolZ mutant. Glucose 6-phosphate uptake at pH 5.5, which is driven by a transmembrane pH gradient, in the tolZ mutant was similar to the parent level. These results indicate that the tolZ mutant has a defect in the generation of delta-mu H+ and delta psi.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein A., Rolfe B., Onodera K. Pleiotropic properties and genetic organization of the tolA,B locus of Escherichia coli K-12. J Bacteriol. 1972 Oct;112(1):74–83. doi: 10.1128/jb.112.1.74-83.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon T. Inactivation of ribosomes in vitro by colicin E 3 . Proc Natl Acad Sci U S A. 1971 Oct;68(10):2421–2425. doi: 10.1073/pnas.68.10.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. M., Dahlberg J. E., Ikemura T., Konisky J., Nomura M. Specific inactivation of 16S ribosomal RNA induced by colicin E3 in vivo. Proc Natl Acad Sci U S A. 1971 May;68(5):964–968. doi: 10.1073/pnas.68.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G., Nordström K. Colicin tolerance induced by ampicillin or mutation to ampicillin resistance in a strain of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):1–13. doi: 10.1128/jb.106.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardelli J., Konisky J. Isolation and characterization of an Escherichia coli mutant tolerant to colicins Ia and Ib. J Bacteriol. 1974 Aug;119(2):379–385. doi: 10.1128/jb.119.2.379-385.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group A. J Bacteriol. 1975 Jul;123(1):102–117. doi: 10.1128/jb.123.1.102-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group B. J Bacteriol. 1975 Jul;123(1):96–101. doi: 10.1128/jb.123.1.96-101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel R. E., Lardy H. A. Stimulation of rat liver mitochondrial adenosine triphosphatase by anions. J Biol Chem. 1975 Jan 10;250(1):191–196. [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G., Nordström K. Genetics and physiology of a tolE mutant of Escherichia coli K-12 and phenotypic suppression of its phenotype by galactose. J Bacteriol. 1973 Sep;115(3):1219–1222. doi: 10.1128/jb.115.3.1219-1222.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman H. R., Helinski D. R. Purification and characterization of colicin E2 and colicin E3. J Biol Chem. 1967 Nov 25;242(22):5360–5368. [PubMed] [Google Scholar]
- Hong J. S., Haggerty D. L., Lieberman M. A. Energy coupling factor as target of colicin K: characterization of a colicin K-insensitive ecf mutant of Escherichia coli. Antimicrob Agents Chemother. 1977 May;11(5):881–887. doi: 10.1128/aac.11.5.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Hunt A. G., Masters P. S., Lieberman M. A. Requirements of acetyl phosphate for the binding protein-dependent transport systems in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1213–1217. doi: 10.1073/pnas.76.3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E. Energy requirement for the initiation of colicin action in Escherichia coli. Biochim Biophys Acta. 1975 Apr 14;387(1):12–22. doi: 10.1016/0005-2728(75)90048-1. [DOI] [PubMed] [Google Scholar]
- Kadner R. J., Winkler H. H. Energy coupling for methionine transport in Escherichia coli. J Bacteriol. 1975 Sep;123(3):985–991. doi: 10.1128/jb.123.3.985-991.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konisky J. Characterization of colicin Ia and colicin Ib. Chemical studies of protein structure. J Biol Chem. 1972 Jun 25;247(12):3750–3755. [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lieberman M. A., Simon M., Hong J. S. Characterization of Escherichia coli mutant incapable of maintaining a transmembrane potential. MetC ecfts mutations. J Biol Chem. 1977 Jun 25;252(12):4056–4067. [PubMed] [Google Scholar]
- Luria S. E. The mistaken identity of colicin A. J Bacteriol. 1982 Jan;149(1):386–386. doi: 10.1128/jb.149.1.386-386.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa H., Hayakawa K., Sato T., Imahori K. Characterization and genetic analysis of a mutant of Escherichia coli K-12 with rounded morphology. J Bacteriol. 1973 Jul;115(1):436–442. doi: 10.1128/jb.115.1.436-442.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: power transmission by proticity. Biochem Soc Trans. 1976;4(3):399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
- Ohno-Iwashita Y., Imahori K. Assignment of the functional loci in colicin E2 and E3 molecules by the characterization of their proteolytic fragments. Biochemistry. 1980 Feb 19;19(4):652–659. doi: 10.1021/bi00545a008. [DOI] [PubMed] [Google Scholar]
- Plate C. A. Mutant of Escherichia coli defective in response to colicin K and in active transport. J Bacteriol. 1976 Feb;125(2):467–474. doi: 10.1128/jb.125.2.467-474.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate C. A. Requirement for membrane potential in active transport of glutamine by Escherichia coli. J Bacteriol. 1979 Jan;137(1):221–225. doi: 10.1128/jb.137.1.221-225.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyssegur J., Stoeber F. Genetic control of the 2-keto-3-deoxy-d-gluconate metabolism in Escherichia coli K-12: kdg regulon. J Bacteriol. 1974 Feb;117(2):641–651. doi: 10.1128/jb.117.2.641-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The relationship between the electrochemical proton gradient and active transport in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):854–859. doi: 10.1021/bi00624a007. [DOI] [PubMed] [Google Scholar]
- Saxe L. S. The action of colicin E2 on supercoiled lambda DNA.II. Experiments in vitro. Biochemistry. 1975 May 20;14(10):2058–2063. doi: 10.1021/bi00681a004. [DOI] [PubMed] [Google Scholar]
- Schaller K., Nomura M. Colicin E2 is DNA endonuclease. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3989–3993. doi: 10.1073/pnas.73.11.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S., Kaback H. R. Membrane potential and active transport in membrane vesicles from Escherichia coli. Biochemistry. 1975 Dec 16;14(25):5451–5461. doi: 10.1021/bi00696a011. [DOI] [PubMed] [Google Scholar]
- Schwartz S. A., Helinski D. R. Purification and characterization of colicin E1. J Biol Chem. 1971 Oct 25;246(20):6318–6327. [PubMed] [Google Scholar]
- Senior B. W., Holland I. B. Effect of colicin E3 upon the 30S ribosomal subunit of Escherichia coli. Proc Natl Acad Sci U S A. 1971 May;68(5):959–963. doi: 10.1073/pnas.68.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague G. F., Jr, Bell R. M., Cronan J. E., Jr A mutant of Escherichia coli auxotrophic for organic phosphates: evidence for two defects in inorganic phosphate transport. Mol Gen Genet. 1975 Dec 30;143(1):71–77. doi: 10.1007/BF00269422. [DOI] [PubMed] [Google Scholar]
- Timmis K., Hedges A. J. The killing of sensitive cells by colicin D. Biochim Biophys Acta. 1972 Mar 14;262(2):200–207. doi: 10.1016/0005-2787(72)90233-x. [DOI] [PubMed] [Google Scholar]
- Timmis K. Purification and characterization of colicin D. J Bacteriol. 1972 Jan;109(1):12–20. doi: 10.1128/jb.109.1.12-20.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H., Konisky J. In vitro depolarization of Escherichia coli membrane vesicles by colicin Ia. J Biol Chem. 1978 Nov 10;253(21):7731–7737. [PubMed] [Google Scholar]
- Tokuda H., Konisky J. Mode of action of colicin Ia: effect of colicin on the Escherichia coli proton electrochemical gradient. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2579–2583. doi: 10.1073/pnas.75.6.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T., Rosen B. P. Characterization of an active transport system for calcium in inverted membrane vesicles of Escherichia coli. J Biol Chem. 1975 Oct 10;250(19):7687–7692. [PubMed] [Google Scholar]



