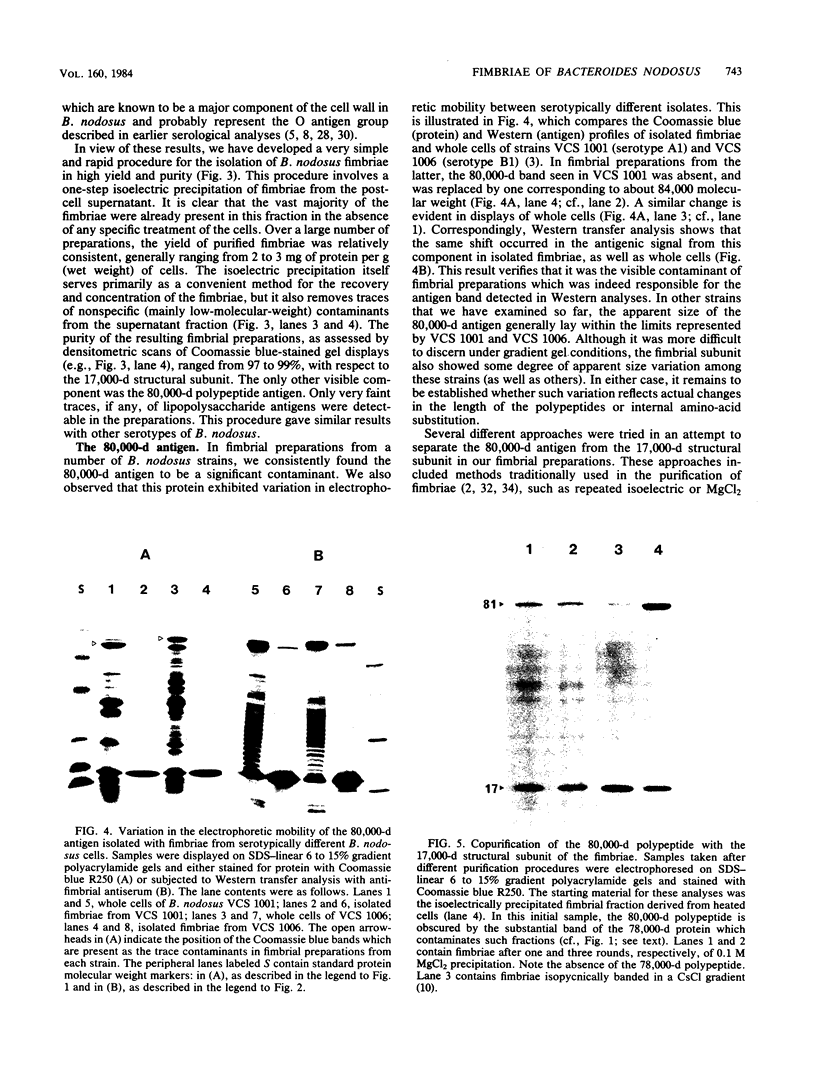
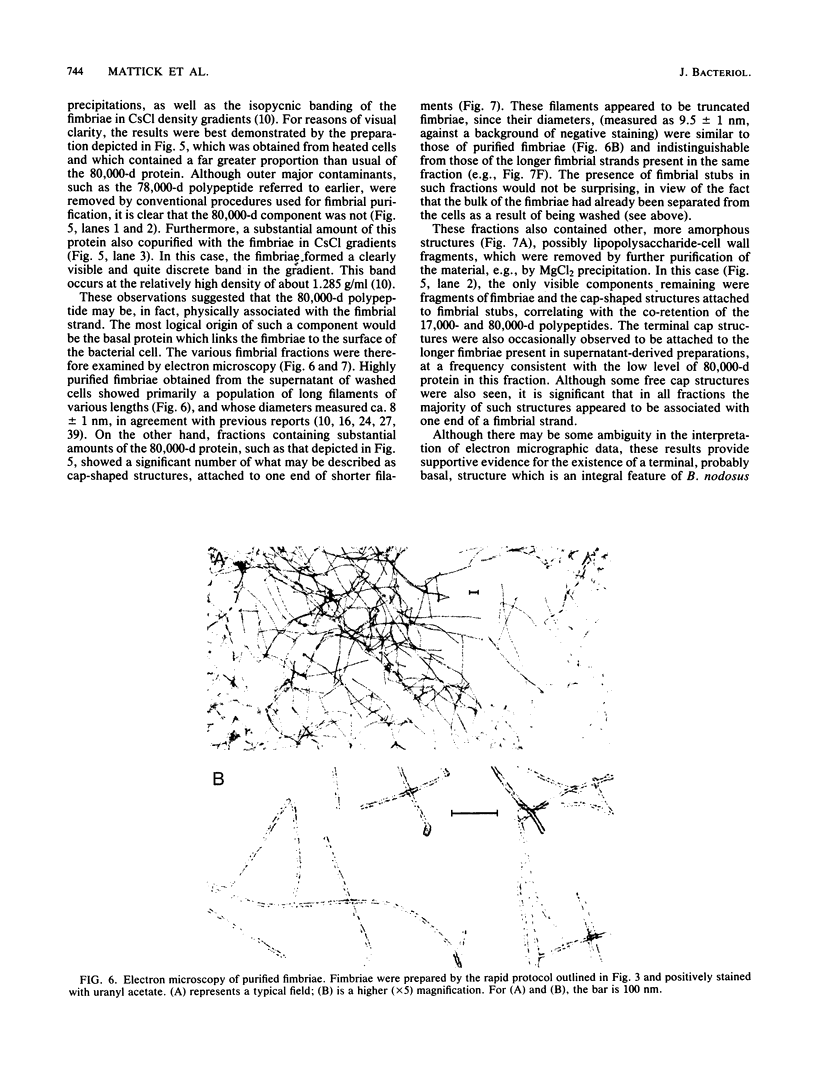
Abstract
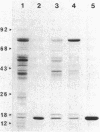
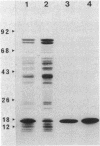
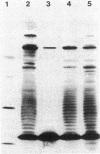
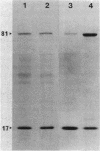
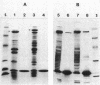
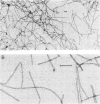
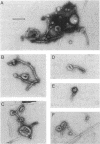
We examined the isolation of fimbriae from Bacteroides nodosus. It was found that the best preparations were obtained from the supernatant of washed cells cultured on solid medium, from which fimbriae could be recovered in high yield and purity by a simple one-step procedure. Analysis of such preparations by sodium dodecyl sulfate gel electrophoresis showed that greater than 98% of the protein consisted of fimbrial structural subunits whose molecular weight was ca. 17,000. These preparations also usually exhibited minor contamination with a polypeptide of ca. 80,000 molecular weight, as well as trace amounts of lipopolysaccharide. Attempts to release additional fimbriae by the traditional means of subjecting the bacterial cells to physical stress, such as shearing or heating, resulted primarily in an increase in the level of contamination, without significant gain in the yield of fimbriae. Removal of the 80,000-dalton component could not be achieved by any of a variety of techniques normally used in fimbriae purification, including isoelectric precipitation, MgCl2 precipitation, and CsCl gradient ultracentrifugation, implying a direct physical association with the fimbrial strand. Electron micrographs of fractions containing this protein show cap-shaped structures attached to the ends of what appeared to be fimbrial stubs. These observations suggest that the 80,000-dalton polypeptide may actually constitute the basal attachment site which anchors the fimbria to the outer membrane, analogous to a similar protein recently described in enterotoxigenic strains of Escherichia coli. In B. nodosus, this 80,000-dalton protein is a major surface antigen, and like the fimbrial subunit, exhibited variation in electrophoretic mobility between serotypically different isolates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Claxton P. D., Ribeiro L. A., Egerton J. R. Classification of Bacteroides nodosus by agglutination tests. Aust Vet J. 1983 Nov;60(11):331–334. doi: 10.1111/j.1751-0813.1983.tb02834.x. [DOI] [PubMed] [Google Scholar]
- Dougan G., Dowd G., Kehoe M. Organization of K88ac-encoded polypeptides in the Escherichia coli cell envelope: use of minicells and outer membrane protein mutants for studying assembly of pili. J Bacteriol. 1983 Jan;153(1):364–370. doi: 10.1128/jb.153.1.364-370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton J. R., Burrell D. H. Prophylactic and therapeutic vaccination against ovine foot-rot. Aust Vet J. 1970 Nov;46(11):517–522. doi: 10.1111/j.1751-0813.1970.tb06636.x. [DOI] [PubMed] [Google Scholar]
- Egerton J. R., Merritt G. C. Serology of foot-rot: antibodies against Fusiformis nodosus in normal, affected, vaccinated and passively immunised sheep. Aust Vet J. 1973 Mar;49(3):139–145. doi: 10.1111/j.1751-0813.1973.tb06762.x. [DOI] [PubMed] [Google Scholar]
- Egerton J. R., Roberts D. S., Parsonson I. M. The aetiology and pathogenesis of ovine foot-rot. I. A histological study of the bacterial invasion. J Comp Pathol. 1969 Apr;79(2):207–215. doi: 10.1016/0021-9975(69)90007-3. [DOI] [PubMed] [Google Scholar]
- Egerton J. R. Significance of Fusiformis nodosus serotypes in resistance of vaccinated sheep to experimental foot-rot. Aust Vet J. 1974 Feb;50(2):59–62. doi: 10.1111/j.1751-0813.1974.tb05252.x. [DOI] [PubMed] [Google Scholar]
- Egerton J. R. Surface and somatic antigens of Fusiformis nodosus. J Comp Pathol. 1973 Jan;83(1):151–159. doi: 10.1016/0021-9975(73)90038-8. [DOI] [PubMed] [Google Scholar]
- Every D. Purification of pili from Bacteroides nodosus and an examination of their chemical, physical and serological properties. J Gen Microbiol. 1979 Dec;115(2):309–316. doi: 10.1099/00221287-115-2-309. [DOI] [PubMed] [Google Scholar]
- Every D., Skerman T. M. Protection of sheep against experimental footrot by vaccination with pili purified from Bacteroides nodosus. N Z Vet J. 1982 Oct;30(10):156–158. doi: 10.1080/00480169.1982.34921. [DOI] [PubMed] [Google Scholar]
- Every D., Skerman T. M. Ultrastructure of the Bacteroides nodosus cell envelope layers and surface. J Bacteriol. 1980 Feb;141(2):845–857. doi: 10.1128/jb.141.2.845-857.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Hamilton R. C., Bover F. G., Mason T. J. An association between fimbriae and pores in the wall of Fusiformis nodosus. J Gen Microbiol. 1975 Dec;91(2):421–424. doi: 10.1099/00221287-91-2-421. [DOI] [PubMed] [Google Scholar]
- Mattick J. S., Zehner Z. E., Calabro M. A., Wakil S. J. The isolation and characterization of fatty-acid synthetase mRNA from rat mammary gland. Eur J Biochem. 1981 Mar;114(3):643–651. doi: 10.1111/j.1432-1033.1981.tb05192.x. [DOI] [PubMed] [Google Scholar]
- Mooi F. R., Wijfjes A., de Graaf F. K. Identification and characterization of precursors in the biosynthesis of the K88ab fimbria of Escherichia coli. J Bacteriol. 1983 Apr;154(1):41–49. doi: 10.1128/jb.154.1.41-49.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. A., Stevens A. E., Sojka W. J. Preliminary characterization of cell-free K99 antigen isolated from Escherichia coli B41. J Gen Microbiol. 1977 Apr;99(2):353–357. doi: 10.1099/00221287-99-2-353. [DOI] [PubMed] [Google Scholar]
- Ottow J. C. Ecology, physiology, and genetics of fimbriae and pili. Annu Rev Microbiol. 1975;29:79–108. doi: 10.1146/annurev.mi.29.100175.000455. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Schmitz J. A., Gradin J. L. Serotypic and biochemical characterization of Bacteroides nodosus isolates from Oregon. Can J Comp Med. 1980 Oct;44(4):440–446. [PMC free article] [PubMed] [Google Scholar]
- Short J. A., Thorley C. M., Walker P. D. An electron microscope study of Bacteroides nodusus: ultrastructure of organisms from primary isolates and different colony types. J Appl Bacteriol. 1976 Jun;40(3):311–315. doi: 10.1111/j.1365-2672.1976.tb04179.x. [DOI] [PubMed] [Google Scholar]
- Skerman T. M. Determination of some in vitro growth requirements of Bacteroides nodosus. J Gen Microbiol. 1975 Mar;87(1):107–119. doi: 10.1099/00221287-87-1-107. [DOI] [PubMed] [Google Scholar]
- Skerman T. M., Erasmuson S. K., Every D. Differentiation of Bacteroides nodosus biotypes and colony variants in relation to their virulence and immunoprotective properties in sheep. Infect Immun. 1981 May;32(2):788–795. doi: 10.1128/iai.32.2.788-795.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward D. J., Egerton J. R. Studies on the ultrastructural morphology of Bacteroides nodosus. Res Vet Sci. 1979 Mar;26(2):227–235. [PubMed] [Google Scholar]
- Stewart D. J. An electron microscopic study of Fusiformis nodosus. Res Vet Sci. 1973 Jan;14(1):132–134. [PubMed] [Google Scholar]
- Stewart D. J. Biochemical and biological studies on the lipopolysaccharide of Bacteroides nodosus. Res Vet Sci. 1977 Nov;23(3):319–325. [PubMed] [Google Scholar]
- Stewart D. J., Clark B. L., Emery D. L., Peterson J. E., Fahey K. J. A Bacteroides nodosus immunogen, distinct from the pilus, which induces cross-protective immunity in sheep vaccinated against footrot. Aust Vet J. 1983 Mar;60(3):83–85. doi: 10.1111/j.1751-0813.1983.tb05877.x. [DOI] [PubMed] [Google Scholar]
- Stewart D. J., Clark B. L., Peterson J. E., Griffiths D. A., Smith E. F. Importance of pilus-associated antigen in Bacteroides nodosus vaccines. Res Vet Sci. 1982 Mar;32(2):140–147. [PubMed] [Google Scholar]
- Stewart D. J. Studies on the antigenic structure of Bacteroides nodosus. Res Vet Sci. 1978 May;24(3):293–299. [PubMed] [Google Scholar]
- Stewart D. J. The role of various antigenic fractions of Bacteroides nodosus in eliciting protection against foot-rot in vaccinated sheep. Res Vet Sci. 1978 Jan;24(1):14–19. [PubMed] [Google Scholar]
- Stirm S., Orskov F., Orskov I., Mansa B. Episome-carried surface antigen K88 of Escherichia coli. II. Isolation and chemical analysis. J Bacteriol. 1967 Feb;93(2):731–739. doi: 10.1128/jb.93.2.731-739.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley C. M. A simplified method for the isolation of Bacteroides nodusus from ovine foot-rot and studies on its colony morphology and serology. J Appl Bacteriol. 1976 Jun;40(3):301–309. doi: 10.1111/j.1365-2672.1976.tb04178.x. [DOI] [PubMed] [Google Scholar]
- Thorley C. M., Egerton J. R. Comparison of alum-absorbed or non-alum-absorbed oil emulsion vaccines containing either pilate or non-pilate Bacteroides nodosus cells in inducing and maintaining resistance of sheep to experimental foot rot. Res Vet Sci. 1981 Jan;30(1):32–37. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. D., Short J., Thomson R. O., Roberts D. S. The fine structure of Fusiformis nodosus with special reference to the location of antigens associated with immunogenicity. J Gen Microbiol. 1973 Aug;77(2):351–361. doi: 10.1099/00221287-77-2-351. [DOI] [PubMed] [Google Scholar]