Abstract
T lymphocytes use LFA-1 to migrate into lymph nodes and inflammatory sites. To investigate the mechanisms regulating this migration, we utilize mAbs selective for conformational epitopes as probes for active LFA-1. Expression of the KIM127 epitope, but not the 24 epitope, defines the extended conformation of LFA-1, which has intermediate affinity for ligand ICAM-1. A key finding is that KIM127-positive LFA-1 forms new adhesions at the T lymphocyte leading edge. This LFA-1 links to the cytoskeleton through α-actinin-1 and disruption at the level of integrin or actin results in loss of cell spreading and migratory speed due to a failure of attachment at the leading edge. The KIM127 pattern contrasts with high-affinity LFA-1 that expresses both 24 and KIM127 epitopes, is restricted to the mid-cell focal zone and controls ICAM-1 attachment. Identification of distinctive roles for intermediate- and high-affinity LFA-1 in T lymphocyte migration provides a biological function for two active conformations of this integrin for the first time.
Keywords: α-Actinin-1, integrin, LFA-1, migration, T lymphocyte
Introduction
T lymphocytes are continually recruited from the circulation into lymph nodes and other tissues in response to stimuli released at sites of injury or infection (von Andrian and Mempel, 2003; Cyster, 2005). This recruitment involves the integrin LFA-1 (CD11a/CD18; αLβ2) acting as a migratory receptor (Hogg et al, 2003; Dustin et al, 2004). If turnover of active to inactive LFA-1 is prevented, then motility decreases both in vivo and in vitro, indicating that conversion between different activity states is essential for lymphocyte migration (Semmrich et al, 2005; Smith et al, 2005). T lymphocytes move rapidly across the vasculature into lymph nodes where they can reach speeds of ∼40 μm/min (Mempel et al, 2004). This ability to migrate is vital for the ultimate success of the immune response as it increases the opportunity for cellular interactions, thereby improving the chance of antigen recognition.
Integrin activation involves an alteration in conformation giving rise to an affinity increase, and adhesion strengthening through receptor clustering (Carman and Springer, 2003; Dustin et al, 2004; Kinashi, 2005). Crystallization of the β3 integrin αvβ3 has revealed details of the conformational changes that integrins undergo following activation (Xiong et al, 2001). A basic model for β2 integrin LFA-1 activation has been developed from structural data, together with observations from electron microscopic studies of isolated integrins and from the use of mAbs that recognize epitopes expressed upon activation (Takagi et al, 2002; Luo et al, 2007). Basically, one conformation of LFA-1 is bent at the junction between the thigh and calf-1 domains of the αL subunit and between EGF-like domains 1 and 2 of the β2 subunit. Upon activation, LFA-1 extends, exposing an epitope recognized by mAb KIM127 that is located in the EGF-like domain 2 at the bend in the β2 subunit (Robinson et al, 1992; Beglova et al, 2002). The extended KIM127+ LFA-1 adopts both closed and open conformations corresponding to intermediate- and high-affinity forms of LFA-1, respectively (Kinashi, 2006; Luo et al, 2007). The epitope recognized by mAb 24 is located in a loop at the top of the I-like domain near the MIDAS site in the β2 subunit (Lu et al, 2001; Kamata et al, 2002), and its expression is characteristic of the high-affinity conformation of LFA-1 (Dransfield et al, 1992b; Lu et al, 2001; Hogg et al, 2002; Kamata et al, 2002). This epitope is postulated to form upon interaction of the α subunit I domain with the β2 subunit I-like domain via a pulling mechanism, giving rise to the open, high-affinity form of integrin (Alonso et al, 2002; Luo et al, 2007).
In order to attach to integrin ligands and migrate, primary T lymphocytes require stimulus from a chemoattractant such as a chemokine. However, for preactivated T lymphoblasts, LFA-1-mediated binding to an ICAM-1-expressing surface is sufficient to cause cell polarization and migration without the need for added chemokines (Dustin et al, 1997; Smith et al, 2003). Previously we have shown that a ‘focal zone' containing high-affinity 24+ LFA-1 is located in the mid-cell region of the polarized T lymphocyte and influences the speed of migration (Smith et al, 2005). To date, little is known of the characteristics of LFA-1 expressed at the leading edge of migrating T lymphocytes. To investigate the nature of these adhesions and compare them with LFA-1 in the focal zone, we have made use of two well-described mAbs, KIM127 and 24, as probes for intermediate- and high-affinity LFA-1. We find that they detect these two forms of active LFA-1 in distinct regions of the migrating cell.
Results
Expression of LFA-1 activation epitopes KIM127 and 24 on T cells
A comparison was made of the expression patterns of LFA-1 activation epitopes recognized by mAbs KIM127 and 24 on T cells migrating on the LFA-1 ligand ICAM-1. Both freshly derived primary T lymphocytes and cultured T lymphoblasts, that are referred to in this study as T cells, were investigated. When analyzed by flow cytometry, the majority of T cells expressed both epitopes (60–95%; n=10), whereas a lower percentage of freshly derived T lymphocytes expressed the epitopes (10–40%; n=4). These percentages correlated with the proportions of cells able to attach to immobilized ICAM-1 (data not shown). Thus, the cells that attach and migrate represent pre-existing subsets on which LFA-1 displays activation epitopes.
The next step was to compare the distribution of the LFA-1 activation epitopes on T cells that were allowed to migrate before fixation and subsequent immunostaining. MAb 24+ LFA-1 was restricted to the mid-cell focal zone both for unstimulated, cultured T cells as previously described (Smith et al, 2005) and primary T lymphocytes that had been stimulated with the chemokine SDF-1 (CXCL12) (Figure 1). In contrast, mAb KIM127 labelled LFA-1 throughout the lamellar region, as well as intensely labelling the focal zone (Figure 1). Therefore, the extended intermediate-affinity form of LFA-1 that expresses the KIM127, but not 24, epitope is localized to the spreading lamella of the T cell, whereas high-affinity LFA-1 that expresses both epitopes is restricted to the focal zone. The distribution patterns of LFA-1 epitopes show that these two conformations of active LFA-1 have distinct locations on the migrating T cell.
Figure 1.
Expression of LFA-1 activation epitopes on migrating T lymphocytes. T cells and freshly isolated primary T lymphocytes migrating on ICAM-1Fc-coated coverslips were fixed and permeabilized before labelling with either KIM127–Alexa546 or 24–Alexa546 addition. Freshly isolated T lymphocytes were exposed for 5 min to SDF-1 (CXCL12) to induce a migratory phenotype. Images are shown in a rainbow false color scale, with the highest expression in red and the lowest blue. The enlarged area of each leading edge is shown with a white box. Scale bar=10 μm.
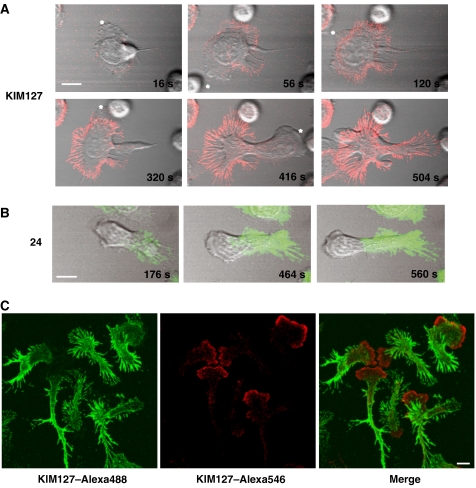
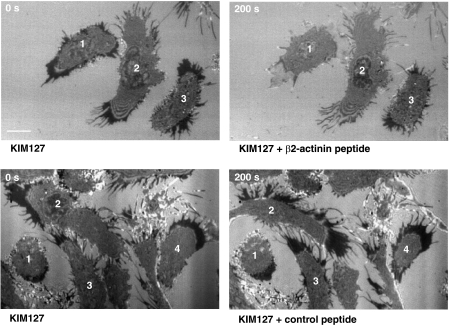
As well as acting as activation reporters, both mAb 24 and KIM127 can stabilize the active forms of LFA-1 that they recognize (Dransfield et al, 1992a; Robinson et al, 1992; Smith et al, 2005; Nishida et al, 2006). When T cells were exposed to mAb KIM127 during migration, KIM127+ LFA-1 attachments (red) to ICAM-1 were formed at the leading edge (see asterisks). As the T cell lamellae extended and made further attachments, KIM127+ staining increased and the T cell rapidly stopped translocating forward (Figure 2A; Supplementary Figure 1 and Video 1). When the KIM127+ adhesions were stabilized to a certain level around one leading edge, another lamellipodial protrusion appeared that was in turn stabilized by making new KIM127+ attachments. Eventually when the leading edge membranes could no longer move in any direction, the cell reversed its polarity and the process was repeated until the T cell was unable to make any new KIM 127+ attachments.
Figure 2.
Effect of mAbs KIM127 and 24 on migrating T cells. (A) Live-cell imaging of T cells migrating on ICAM-1Fc-coated coverslips following addition of directly labelled mAb KIM127 at 0 s. Confocal images showing KIM127–Alexa546 (red) localization are overlaid with the phase images. The leading lamellipodia are indicated by *. Scale bar=10 μm. (B) Images as in panel A but following addition of 24–Alexa488 (green) at 0 s. (C) Addition of KIM127–Alexa488 (green) for 5 min before counterstaining with KIM127–Alexa546 (red) during fixation. Scale bar=5 μm.
By comparison, when T cells were exposed to mAb 24 during migration, 24+ LFA-1 (green) was focused not at the leading edge but in the focal zone (Figure 2B; Supplementary Figure 1). Over time the focal zone extended forward as the number of new 24+ attachments increased. Of note is the observation that KIM127 does not have access to its epitope in the focal zone area as might be expected. However, if the migrating cell is subsequently fixed and then stained for KIM127, the epitope is detected in the focal zone in keeping with the pattern of immunostaining shown in Figure 1 (data not shown). There may be a lack of access of KIM127 in the focal zone region because of the positioning of the epitope on LFA-1 or because packing of LFA-1 molecules in this region interferes with mAb binding.
To investigate the formation of KIM127+ adhesions in more detail, we carried out a two-stage experiment focusing on the interface with ICAM-1. T cells were allowed to migrate in an excess amount of KIM127-Alexa488 (green) to label the available adhesions. Unbound mAb was then washed out, and, to capture the adhesions formed subsequently, KIM127-Alexa546 (red) was added while the cells were being fixed. Under these conditions, KIM127+ adhesions (red) were only observed at the leading edge confirming that new KIM127+ adhesions form in this location and are stabilized by the mAb (Figure 2C). This stabilizing effect then causes the unattached leading edge of the T cell to move in another direction over pre-existing stabilized LFA-1 adhesions to form new ones.
We next used interference reflection microscopy (IRM) to focus on the interface between LFA-1 on migrating T cells and ICAM-1 at the leading edge following addition of KIM127. At 0 s the T cell migrates in one direction, with its leading edge indicated by an arrow (Figure 3; Supplementary Video 2). After 30 s of exposure to KIM127, LFA-1 at the leading edge has become stabilized. The LFA-1 attachment points are seen as dark contrast regions and a new leading edge moves the cell in another direction. At 70 s, the cell changes direction again as the second leading edge becomes stabilized. Finally at 130 s the LFA-1 adhesions are stabilized all around the cell and it has come to a halt. We conclude that mAb KIM127 prevents turnover of LFA-1/ICAM-1 attachments through its ability to stabilize LFA-1 adhesions at the leading edge. Addition of mAb 24 has no effect on the leading edge (data not shown).
Figure 3.
Effect of mAb KIM127 on IRM imaging of a T cell migrating on ICAM-1. The image of a single representative T cell migrating on an ICAM-1-coated coverslip is shown with 10 μg/ml KIM127 added at 0 s. KIM127 stabilizes adhesions of leading edge 1 (see arrow) at 30 s shown by the formation of dark contact areas. The cell alters direction, forming leading edge 2, which also becomes stabilized (70 s). This is followed by stabilization of leading edge 3 (130 s) and the process continues until the cell can no longer move. Scale bar=5 μm.
In contrast to both the 24+ and KIM127+ adhesions, the majority of which are restricted to LFA-1 in contact with ICAM-1, total LFA-1 was distributed over the entire cell surface (Supplementary Figure 2). The lamella at the leading edge displayed a lower level of LFA-1 compared with the focal zone and the uropod at the rear as previously reported (Smith et al, 2005). Therefore, for T cells migrating on ICAM-1, the presence of mAbs recognizing activation epitopes causes build up of LFA-1 adhesions at the leading edge for KIM127, and at the focal zone, for 24.
A role for intermediate-affinity LFA-1 in random T cell migration and chemotaxis
As mAbs 24 and KIM127 labelled distinct LFA-1 conformations in different locations when T cells were migrating on ICAM-1, we asked whether the way in which these mAbs affected migration could provide information about the differing roles of the two forms of LFA-1. Non-blocking anti-LFA-1 mAb YTH 81.5 had no effect, but KIM127 caused a 63±4% reduction in speed of migration (Figure 4A) by preventing the translocation of individual T cells (Figure 4B). In contrast, there was no significant alteration in cell speed after the addition of 24 for the initial 10-min period (Figure 4A and B), but longer exposure gradually resulted in reduced migration (data not shown). To recreate more closely a potential in vivo situation, the effect of these mAbs on T cell migration through ICAM-1-coated filters in response to chemokine SDF-1 was tested (Figure 4C). SDF-1-induced migration was inhibited 96.4±0.7% by KIM127, 45.7±1.0% by 24 and was unaffected by YTH 81.5 (Figure 4C).
Figure 4.
Effect of KIM127 and 24 mAbs on T cell migration and chemotaxis. (A) Speed of migration of T cells on ICAM-1Fc-coated coverslips in the first 10 min following addition of LFA-1 mAbs YTH81.5, KIM127 or 24. Migration speed was calculated for 10 cells per treatment and expressed as a percentage of control cells. Data from a representative experiment are shown (n=3; *P<0.01). (B) Migratory tracks of individual T cells in the presence of mAbs YTH81.5, KIM127 or 24 from the same experiment as in panel A. (C) T cell chemotaxis for 60 min across ICAM-1Fc-coated filters in response to 10 nM SDF-1 in the presence or absence of the indicated LFA-1 mAbs. Each condition was run in duplicate. A representative experiment (n=3) is shown. (D) Live-cell imaging of T cells migrating on TNF α-activated-HUVECs following addition of fluorescently labelled mAbs (0–180 s). Confocal images of KIM127 (red) or 24 (green) localization are overlaid with phase images. The leading edge is indicated by * and shedding of 24-positive LFA-1 from the trailing edge by white arrows. Scale bar=10 μm.
Confocal microscopy was used to observe T cells migrating on TNF-α-activated human umbilical vein endothelial cells (HUVECs) following exposure to fluorescently labelled KIM127 or 24 (Figure 4D; Supplementary Videos 3A and B). The cells that bound KIM127 failed to translocate, showing that KIM127 effectively inhibited turnover of LFA-1 adhesions to the endothelial cell surface. In contrast, mAb 24 stabilized only the mid-cell focal zone LFA-1, leaving the leading edge free to make fresh attachments and move forward. In fact the strength of this motility was sufficient to overcome the 24-stabilized LFA-1 adhesions, a proportion of which were left behind on the endothelium (see arrows). These results further underline the importance of intermediate-affinity KIM127+ LFA-1 in making adhesions at the front of the cell and show that this LFA-1 controls the ability of T cells to migrate.
KIM127+ LFA-1 at the T cell leading edge is linked to α-actinin-1
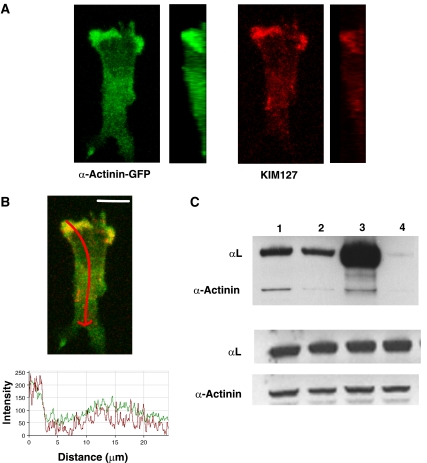
Given the dependence of LFA-1-mediated migration on reorganization of the cytoskeleton (Porter et al, 2002; Smith et al, 2003) and the dramatic effect of KIM127 on T cell migration, an issue was how intermediate-affinity LFA-1 might be linked into the cytoskeleton. Pavalko and co-workers have demonstrated binding of the neutrophil β2 integrin subunit to both talin and α-actinin (Sampath et al, 1998). As the focal zone LFA-1 is associated with talin (Smith et al, 2005), we explored whether α-actinin-1 might be associated with KIM127+ LFA-1. The T cell line HSB-2 was transfected with α-actinin-1–GFP (Guvakova et al, 2002) and the migrating cells exposed to mAb KIM127 while being fixed so that the mAb would label the newest adhesions. α-Actinin-1 was chiefly localized at the leading edge of the cell (green), overlapping with KIM127+ LFA-1 (red) (Figure 5A and B). Quantification of the expression levels confirmed that the highest levels of both molecules were at the leading edge (Figure 5B). When viewed as a vertical slice along the y-axis, it was apparent that colocalized KIM127+ LFA-1 and α-actinin-1 were not restricted to the interface with ICAM-1, but could also be found on non-attached membrane at the front of the cell (Figure 5A). As a control, GFP transfected into T cells was evenly distributed, indicating that the α-actinin–GFP pattern did not simply represent the volume occupied by ruffled membranes (data not shown).
Figure 5.
KIM127+LFA-1 binds to cytoskeletal protein α-actinin-1 at the leading edge of T cells. Confocal images of a representative HSB-2 cell transfected with α-actinin-1-GFP migrating on ICAM-1Fc and stained with KIM127–Alexa546 during fixation. (A) Z-stack and vertical slice along the y-axis of α-actinin–GFP (green) and KIM127+LFA-1-labelled (red) HSB-2 cells are shown. (B) Merged z-stack of α-actinin-GFP (green) and KIM127+LFA-1 (red) images. The level of expression of α-actinin-1 (green trace) and KIM127+ LFA-1 (red trace) along the length of the polarized HSB-2 T cell (indicated by red arrow on merged image) is recorded. Scale bar=10 μm. (C) Co-immununoprecipitation of αL and α-actinin-1. T cells were preincubated with primary mAb before lysis and western blotting. Top panel shows lysates of T cells preincubated with the following: lane 1, KIM127; lane 2, 24; lane 3, total LFA-1 mAb 38 and lane 4, control mAb 52 U. Western blotting using αL and α-actinin-1 mAbs shows the extent of co-immunoprecipitation. Middle and bottom panels show total αL and α-actinin-1 levels, respectively, from 10 μl of lysate (n=4).
Further evidence for an association between LFA-1 and α-actinin-1 was sought by co-immunoprecipitation of T cell proteins using mAbs KIM127 and 24, pan-LFA-1 mAb 38 and an isotype control mAb (Figure 5C). α-Actinin-1 co-immunoprecipitated with KIM127+ and 38+ LFA-1, whereas the co-immunoprecipitation of α-actinin-1 with 24+ LFA-1 was at background levels (top panel). Loading control lanes for the LFA-1 αL subunit and α-actinin-1 are shown in the bottom panels. Using densitometry, we estimated that mAbs KIM127 and 38, but not mAb 24 or control mAb, immunoprecipitated 0.45±0.1% of total α-actinin-1 (n=3). The α-actinin-1 associated with LFA-1 therefore represents a small but distinctive subpopulation of this cytoskeleton-associated protein. Together, these results provide evidence that intermediate-affinity LFA-1 on T cells is associated with α-actinin-1 at the leading edge.
T cell migration on ICAM-1 requires α-actinin-1
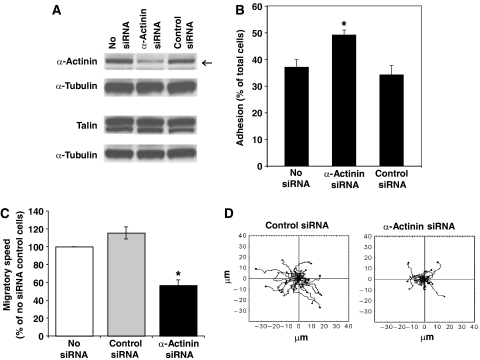
We asked whether α-actinin-1 was required for T cell migration and also in the step before migration that involves cell attachment from suspension onto immobilized ICAM-1. Small interfering RNA (siRNA) directed against α-actinin-1 was transfected into the HSB-2 T cell line, resulting in a 60% knockdown after 48 h (see arrow), but with no effect on talin expression (Figure 6A). The decreased level of α-actinin-1 did not impede T cell attachment and, in fact, caused a 35±5% increase in the level of adhesion (Figure 6B). However, when migration on ICAM-1 was assessed, α-actinin-1-knockdown T cells showed a 53±5% reduction in speed and a decreased ability of individual cells to translocate compared with control siRNA-transfected or untransfected cells (Figure 6C and D). These results are in contrast to knockdown of talin by 40% that produced a corresponding decrease in HSB-2 T cell attachment (Supplementary Figure 3), suggesting a key role for talin, and not α-actinin-1, in this step. Therefore α-actinin-1 has no role in the initial attachment to ICAM-1, but does have a major impact on the ability of the T cells to migrate.
Figure 6.
Effect of α-actinin-1 knockdown on T cell migration and adhesion on ICAM-1. (A) Western blot of total HSB-2 cell lysates from the following transfection steps: no siRNA, α-actinin-1 siRNA (ID 9416) or control siRNA transfectants probed for α-actinin-1, talin and α-tubulin. The level of α-actinin-1 knockdown was ∼60% (n=3). Two alternative siRNAs to α-actinin-1 (ID 147017 or ID 16804) knocked down by ∼30% (data not shown). (B) Adhesion to ICAM-1 of HSB-2 T cells transfected with no siRNA, control siRNA or α-actinin-1 siRNA (*P<0.01). The cells shown were from the same transfection as in panel A (representative experiment of n=3). (C) Average speed of HSB-2 cells transfected with no siRNA, control siRNA or α-actinin-1 siRNA (36 cells each; mean values of n=3; *P<0.01). (D) Cell tracks of migrating HSB-2 T cells transfected with control siRNA or siRNA specific for α-actinin-1 from the same data as in panel C and tracked for 10 min (12 cell tracks per condition).
α-Actinin-1 links to the actin cytoskeleton during T cell migration
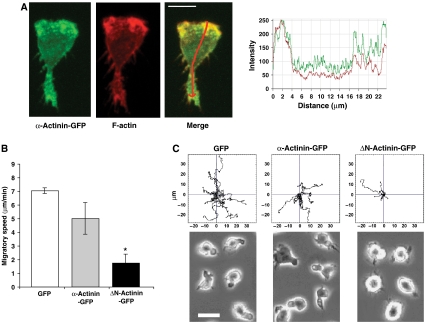
To establish whether α-actinin-1 was linked to the actin cytoskeleton, we transfected HSB-2 T cells with α-actinin–GFP (green) and detected F-actin with phalloidin–Alexa546 (red). There was overlap at the leading edge between the highest cellular levels of α-actinin-1 and F-actin (Figure 7A). Next, T cells were transfected with an α-actinin-1 construct (α-actinin–GFP), a second α-actinin-1 construct lacking the actin-binding domain (ΔN-actinin–GFP) or GFP as a control. The T cells expressing ΔN-actinin–GFP showed a 75±9% decrease in speed compared with α-actinin–GFP and control GFP-expressing cells (Figure 7B). Tracking individual cells highlighted a loss of translocation associated with the ΔN-actinin–GFP compared with controls (Figure 7C). The cells expressing α-actinin–GFP or GFP had a polarized morphology, whereas the ΔN-α-actinin–GFP transfectants attached normally to ICAM-1, but remained rounded (Figure 7C). Therefore colocalization of α-actinin-1 and F-actin at the leading edge, as well as the effect of the α-actinin-1 construct lacking the actin-binding domain, indicated the critical role played by α-actinin-1 linkage to the actin cytoskeleton in T cell morphology and migration.
Figure 7.
KIM127+ LFA-1 requires attachment to the actin cytoskeleton via α-actinin-1 for spreading and migration on ICAM-1. (A) Confocal image of a representative HSB-2 cell transfected with α-actinin–GFP (green) and counterstained with Alexa546–phalloidin to detect F-actin (red) following fixation. The level of expression of α-actinin–GFP (green trace) and F-actin staining (red trace) along the length of the polarized T cell (indicated by red arrow in merged image) is recorded. Scale bar=10 μm. (B) Average speed of HSB-2 T cells transfected with either GFP, α-actinin–GFP or ΔN-actinin–GFP (α-actinin-1 mutant missing the actin-binding domain) (mean values of n=3; 36 cells per condition; *P<0.01). (C) Cell tracks and video microscopy images of HSB-2 transfectants as in panel B; 12 cells tracked per condition. Representative experiment of n=3. Scale bar=10 μm.
α-Actinin-1 binding to the LFA-1 β2 subunit is necessary for T cell migration
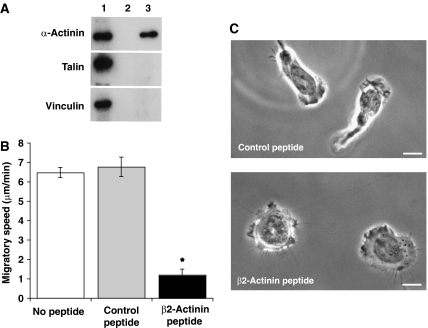
The previous experiments showed that α-actinin-1 interacting with F-actin at the leading edge was required for successful T cell migration and that a sub-population of α-actinin-1 is associated with KIM127+ LFA-1. A question was whether the LFA-1/α-actinin-1 association was direct and whether interfering with it would alter aspects of migration that were also altered by manipulating α-actinin-1 itself. To investigate the interaction between LFA-1 and α-actinin-1, we synthesized a cell-permeable peptide corresponding to the α-actinin-1-binding site (H728–S745) (β2-actinin peptide) on the LFA-1 β2 subunit cytoplasmic tail (Pavalko and LaRoche, 1993). In a pull-down assay comparing the β2-actinin peptide (lane 3) with the scrambled control peptide (lane 2), the β2-actinin peptide showed selectivity in binding α-actinin-1 but not talin or vinculin (Figure 8A).
Figure 8.
Effects of β2-actinin-1 peptide on T cell adhesion, migration and morphology on ICAM-1. (A) Western blot of the following is shown: lane 1, total T cell lysate; lane 2, pull down using a biotin-labelled control peptide; lane 3, pull down using biotin-labelled β2-actinin peptide probed for α-actinin-1, talin and vinculin. (B) Average speed of migration of T cells preincubated without peptide, with control peptide or β2-actinin peptide (mean values of n=3; 24 cells per condition). (C) Video microscopy images of T cells preincubated for 30 min with 50 μg/ml control or β2-actinin peptide. Scale bar=10 μm.
T cells were then preincubated with the two peptides to test their effect on migration. The speed of β2-actinin peptide-treated cells was reduced by 87±4%, with the control peptide-treated cells being similar to the untreated cells (Figure 8B). When viewed by real-time low-light microscopy, β2-actinin peptide-treated cells were attached but exhibited dynamic membrane activity, with projections extending and retracting around the perimeter of the cell (Figure 8C). Therefore, the failure to migrate was not due to an inability to generate membrane protrusions (Supplementary Video 4A). Control peptide-treated cells migrated normally (Figure 8C; Supplementary Video 4B).
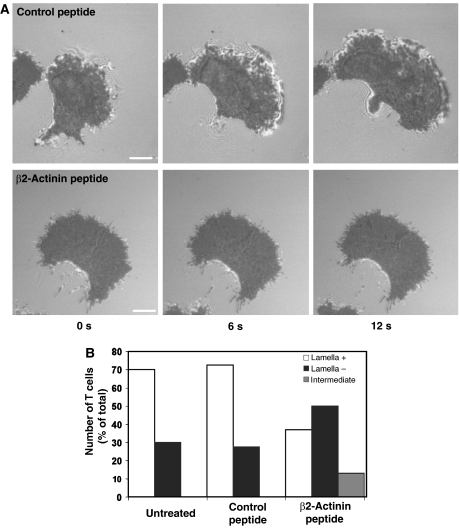
We used IRM to examine in more detail the close contacts with ICAM-1 made by migrating T cells following preincubation with peptide. On untreated (data not shown) and control peptide-treated cells, the lamellar membranes displayed dynamic patterns of intermittent attachment in the direction of cell movement over time, as well as a more uniform darker contact area that followed behind the leading edge (Figure 9A). In contrast, the β2-actinin peptide-treated T cells showed only the dark close contact area, with little evidence for membranes making new contacts over time (Figure 9A). Analysis of cell morphology over 30 s showed that ∼70% of control peptide and untreated cells displayed a defined leading edge comprising lamella and lamellipodium, whereas 50% of the β2-actinin peptide-treated cells had no definite leading edge, and a further 10% showed minimal lamellipodial extension (Figure 9B). The images indicate that exposure of migrating T cells to a peptide designed to disrupt the LFA-1/α-actinin-1 connection interferes with the ability of the cell to generate or to maintain the contacts with ICAM-1 that lead to spreading at the leading edge.
Figure 9.
Effect of β2-actinin-1 peptide on the IRM image of migrating T cells. (A) IRM images of representative T cells treated with control scrambled peptide or β2-actinin peptide for 30 min before migrating on ICAM-1. The three frames show images recorded at 0, 6 and 12 s. Images are representative of 40 cells per treatment group (n=3). Scale bar=5 μm. (B) Analysis of IRM images of T cells treated with β2-actinin or control peptide. The IRM ‘bright' image for each cell was scored as extensive (lamella+), partial (intermediate) or absence of obvious lamella/lamellipodial region (n=40 for each group in two experiments).
KIM127+ LFA-1-stabilized adhesions to ICAM-1 are disrupted by the β2-actinin peptide
The β2-actinin peptide had an inhibitory effect on LFA-1 attachments to ICAM-1, but we wanted to explore whether or not it was KIM127+ LFA-1 linked to α-actinin that was involved in making the adhesions at the leading edge. KIM127 mAb was added to migrating T cells and IRM analysis showed that the LFA-1 adhesions were stabilized as shown in Figure 3 (Figure 10). We then added either β2-actinin or control peptide to explore any effect on the KIM127+ adhesions. Over 200 s, the dark contrast KIM127-stabilized LFA-1 adhesions diminished in the β2-actinin peptide-treated T cells (Figure 10; Supplementary Video 5A). In contrast, there was no effect on KIM127 adhesions with control peptide (Figure 10; Supplementary Video 5B) or untreated cells (data not shown). We conclude that it is intermediate-affinity KIM127+ LFA-1 that forms the critical attachment to α-actinin at the leading edge essential for spreading and migration of the T cell.
Figure 10.
Effect of β2-actinin peptide on the IRM image of migrating T cells following treatment with KIM127. IRM images are shown of T cells that are pretreated with 10 μg/ml KIM127, resulting in KIM127-stabilized adhesions (see dark contact areas). The cells are then treated with either β2-actinin or control peptide and the IRM image recorded after 200 s. The β2-actinin peptide caused the KIM127 stabilized adhesions to diminish, whereas the control peptide had no effect. Scale bar=10 μm.
FRET analysis shows proximity between LFA-1 and α-actinin-1 at the leading edge
As well as binding to α-actinin-1, the β2 subunit also binds to talin and shares the talin-binding site with phosphatidylinositol phosphate kinase type 1γ (PIPK1γ) (S745–T758) (de Pereda et al, 2005). In a recent publication, a second talin-binding site has been mapped on the β subunit that, together with the PIPK1γ site, supports the activation of integrin (Wegener et al, 2007). This newly identified sequence incorporates the α-actinin-1-binding site and raises the possibility that the β subunit might bind α-actinin-1 and talin sequentially. Our previous results showed that LFA-1 interacted with talin in the mid-cell focal zone region (Smith et al, 2005). We therefore used fluorescence resonance energy transfer (FRET) to ask first whether we could detect any close contact between LFA-1 and α-actinin-1 in the lamella, focal zone or uropod regions on migrating T cells and, secondly, whether the region of the β2 cytoplasmic tail covered by the β2-actinin peptide served as the binding site for α-actinin-1 in the migrating cell.
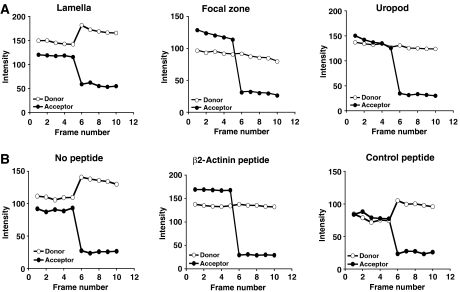
Acceptor depletion FRET (adFRET) is a variation of the FRET technique, where an increase in donor signal following photobleaching of acceptor indicates that FRET had been occurring before the photobleaching step, but was quenched by the acceptor of donor fluorescence (Di et al, 2005). We asked whether an adFRET signal could be detected between LFA-1 containing a GFP-tagged β2 subunit and endogenous α-actinin-1 labelled with Alexa546-conjugated anti-α-actinin-1 mAb. GFP–LFA-1 displayed a positive FRET signal, with α-actinin-1 at the leading edge of the HSB-2 T cells that was not detected either in the focal zone or the uropod (Figure 11A). This result indicates that LFA-1 is within 1–10 nm of α-actinin-1 at the leading edge, but not in the other two compartments of the migrating cell.
Figure 11.
FRET analysis showing association between LFA-1 and α-actinin-1 at the leading edge of HSB-2 T cells. (A) AdFRET profile showing fluorescence intensity of acceptor Alexa546-labelled α-actinin-1 and donor GFP-LFA-1 for five frames before and five frames after photobleaching of the acceptor (n=10 for each zone tested). The increase in fluorescence intensity of donor LFA-1 occurs at the leading edge in the lamella of the migrating T cell but not in the focal zone or uropod. (B) AdFRET profiles at the leading edge were determined as in panel A for cells treated with β2-actinin peptide, control peptide or no peptide. No FRET was seen with addition of β2-actinin peptide, showing that LFA-1 does associate with α-actinin-1 through the β2 cytoplasmic tail sequence covered by the β2-actinin peptide.
To further confirm that the β2-actinin peptide blocked LFA-1 binding to α-actinin-1 at the front of the cell (Pavalko and LaRoche, 1993), we tested its effect on the adFRET signal. GFP–LFA-1-expressing HSB-2 cells were pretreated with either the β2-actinin or scrambled control peptides or no peptide. The β2-actinin peptide was found to abrogate the FRET signal that was otherwise detectable in both untreated or control peptide-treated T cells (Figure 11B). These observations provide additional evidence that LFA-1 at the leading edge of the T cell migrating on ICAM-1 is directly engaged with α-actinin-1, and that the sequence on the β2 subunit (H723–S742) covered by the β2-actinin peptide is where this interaction occurs.
Discussion
In this study we show that intermediate-affinity LFA-1 is expressed throughout the lamella of T cells migrating on ICAM-1 randomly or in response to a chemokine. These LFA-1 adhesions are characterized by expression of the KIM127 epitope, but not the 24 epitope, and are initiated at the leading edge. Exposure of T cells to mAb KIM127 during migration stabilizes the LFA-1 adhesions and effectively brings the cell to a halt, showing that intermediate-affinity LFA-1 plays a dominant role in T cell migration. Previous IRM studies using T cells migrating on ICAM-1 embedded in a lipid bilayer indicate that the LFA-1 found in this zone can comprise up to 80% of the contact with ligand ICAM-1 (Smith et al, 2005).
α-Actinin-1 has a number of functions in migrating cells (Otey and Carpen, 2004). By using siRNA knockdown and competitive inhibition with a mutated α-actinin-1 construct, we show that its ability to bind to actin is essential for T cell migration. The treated T cells remain attached to ICAM-1 in a talin-dependent manner, but fail to project membranes or spread. This is in keeping with a role for α-actinin-1 as an actin-bundling protein, a major function in other cell types (Otey and Carpen, 2004).
We have however focused on the subset of α-actinin-1 that binds to LFA-1 and show by colocalization and immunoprecipitation experiments that KIM127-expressing LFA-1 associates with α-actinin-1. A link between the β subunit of β1, β2 and β3 integrins and α-actinin-1 has been documented previously and the binding sequence identified (Pavalko and LaRoche, 1993; Otey and Carpen, 2004). The structure of the site on the β1 integrin cytoplasmic tail where α-actinin-1 binds has been determined and confirms the importance of this sequence (Kelly and Taylor, 2005).
We show in several ways that binding of KIM127+ LFA-1 to α-actinin-1 has functional importance for T cell migration. T cells fail to migrate following knockdown of α-actinin-1 or more specific targeting of the binding site on the β2 subunit for α-actinin-1 (H723–S742) with a peptide. FRET experiments revealed that this interaction occurs at the leading edge of the migrating T cell. Where LFA-1 binding to α-actinin-1 is prevented, the cells fail to attach and spread, although the T cell membrane continues to protrude. IRM images show reduced lamellipodial attachments in the presence of the LFA-1/α-actinin-1-blocking peptide. A direct functional link between KIM127+ LFA-1 and α-actinin-1 is provided by showing that the blocking peptide can disrupt the KIM127-stabilized adhesions. Thus, intermediate-affinity LFA-1 attachment to α-actinin-1 either generates or stabilizes LFA-1 adhesions that support spreading at the leading edge of the T cell. However, as the cells are more highly adherent when α-actinin-1 is knocked down, it is possible that the protein is needed for the turnover of established adhesions.
In fibroblasts, α-actinin-1 is present at the leading edge of the cell but not included in nascent adhesions containing small integrin clusters (Laukaitis et al, 2001; von Wichert et al, 2003; Wiseman et al, 2004). When α-actinin-1 is incorporated into these adhesions, a more mature focal complex is formed that aids the generation of force necessary for spreading and subsequent migration (von Wichert et al, 2003). This is in keeping with the idea that forward membrane protrusion is dependent upon turnover of adhesions at the leading edge to support strong propulsive forces (Beningo et al, 2001). Further investigation of events at the leading edge of T cells is needed to decide how close the parallels are with the spreading dynamics of fibroblasts.
Confocal and IRM images show that lamellar LFA-1 expressing the KIM127 epitope is in contact with ICAM-1. However, KIM127+ LFA-1 is also observed on the apical surface at the leading edge under conditions of rapid fixing of the T cells. A possibility is that KIM127+ LFA-1 might be released onto the membrane, potentially from intracellular vesicles (Fabbri et al, 2005). Another option is that there may be turnover and reuse of KIM127+ LFA-1/α-actinin-1 adhesions during spreading. Small mobile complexes of β1 integrin and α-actinin-1 are detected on non-attached membranes of fibroblasts (Wiseman et al, 2004).
Another issue is whether the extended conformation of LFA-1 characterized by KIM127 expression is caused by association with α-actinin-1. We find that neither knockdown of α-actinin-1 (or talin) nor incubation with the β2-actinin peptide alters KIM127 expression on migrating T cells, suggesting that α-actinin-1 binds to β2 integrin as a secondary event following integrin activation (data not shown). These findings concur with those of Pavalko and co-workers who postulated that when neutrophils were stimulated, a conformational change occurs on the β2 subunit, revealing the α-actinin-1-binding site (Sampath et al, 1998). This sequence of events is also in agreement with the results of Kinashi and co-workers who have evidence that LFA-1 activation through the Rap1 GTPase pathway induces KIM127+ LFA-1. Active Rap1V12 promotes KIM127 expression while dominant negative Rap1N17 blocks the expression induced by chemokine (T Kinashi, personal communication). Together, the data indicate that binding of LFA-1 to α-actinin-1 is secondary to its conversion from a bent to an extended conformation exposing the KIM127 epitope.
Intermediate-affinity LFA-1 is expressed at the leading edge of the cell and bound to α-actinin-1, whereas high-affinity LFA-1 bound to talin is located in the mid-cell focal zone (this study; Smith et al, 2005). The integrin β subunit shares a well-defined binding site on the talin FERM domain with PIPK1γ (de Pereda et al, 2005). Now a second talin site on the β subunit has been mapped (Wegener et al, 2007), which overlaps the α-actinin-1-binding sequence (H723–S742) on the LFA-1 β2 subunit (Pavalko and LaRoche, 1993). We made use of this sequence to make the β2-actinin peptide and confirmed that it is used for α-actinin-1 binding by means of affinity chromatography and also FRET analysis, showing that the peptide interferes with the signal between LFA-1 and α-actinin-1. Therefore, this site on the β2 subunit may be used both by α-actinin-1, talin and potentially other cytoplasmic proteins, and there may be competition for occupancy. Pavalko and co-workers have suggested that the β2 subunit undergoes alternate cytoskeletal connections to talin and α-actinin-1 (Sampath et al, 1998). Using immunoprecipitation, they found that the neutrophil β2 integrin cytoplasmic tail was associated first with talin, but following neutrophil stimulation, talin binding was lost due to calpain cleavage and an α-actinin-1-binding site was revealed. There is however no information yet as to whether LFA-1 exchanges α-actinin-1 for talin or vice versa as the T cell migrates. It is also possible that other cytoskeletal proteins might be involved. For example, vinculin contains a binding site used by both talin and α-actinin-1 (Kelly et al, 2006). As α-actinin-1 and talin are most highly concentrated in distinct compartments, it may be this spatial arrangement that determines the specific LFA-1 conformation associated with each cytoskeletal protein.
Insight into how the two active forms of LFA-1 function together to enable T cell migration comes from the stabilizing effect of the conformation-specific mAbs 24 and KIM127. The high-affinity 24+ focal zone LFA-1 provides adhesive stability by making firm attachments in the mid-cell region. Such anchoring would support the intermediate-affinity KIM127+ LFA-1, making more dynamic attachments, while the T cell scans a DC cell surface for antigen or an endothelial surface for an exit point through the vascular endothelium. Such a division of roles is also suggested by the behavior of neutrophils migrating through endothelium, where the leading edge of the neutrophil penetrates the cell layer leaving high levels of LFA-1 in a ring-like structure at the trailing edge on the endothelial surface (Shaw et al, 2004).
It is of interest that expression of intermediate-affinity LFA-1 at the leading edge of the T cell differs from migrating endothelial cells that display high-affinity αvβ3 in this location (Kiosses et al, 2001). Such an arrangement of high-affinity integrin at the leading edge might not be suitable for a fast moving T cell that needs to turn over membrane attachments rapidly, but might benefit a slower moving cell by allowing it to make firm contact with its surroundings as it adjusts its position within a particular tissue.
In summary, we have used mAbs specific for conformational epitopes as probes for intermediate- and high-affinity LFA-1 on migrating T cells. This approach has revealed for the first time that these two forms of active LFA-1 are expressed in two different regions of the migrating cell where they are associated with distinct cytoskeletal proteins and perform separate roles. These conformers of LFA-1 are also observed on T cells migrating on two other LFA-1 ligands, ICAM-2 and ICAM-3 (data not shown), and also on endothelial cells, indicating that they are also relevant in a more physiological context. The distinctive cellular locations for different forms of active LFA-1 would not be predicted from the total LFA-1 pattern that shows low-level expression throughout the lamellar region, with much higher levels in the focal zone and the projecting uropod (this study; Smith et al, 2005). This study highlights the flexibility of integrins as receptors in terms of their ability to modulate their affinity in accordance with function and independently of expression on the cell membrane.
Materials and methods
Antibodies and reagents
The following mAbs were used in this study: KIM127 (CD18 activation reporter) (Robinson et al, 1992); YTH81.5 (CD11a non-function blocker) (Smith et al, 2005); 38 (CD11a function blocker) and 24 (CD18 integrin activation reporter) (Dransfield et al, 1992a; Hogg et al, 2002) produced at Cancer Research UK; BM-75.2 (α-actinin-1), 8d4 (talin), hVIN-1 (vinculin) and DM 1A (α-tubulin) were purchased from Sigma-Aldrich, Dorset, UK; AlexaFluor546–phalloidin, AlexaFluor488- and AlexaFluor546-goat anti-mouse IgG were from Invitrogen, Paisley, UK; AlexaFluor488 and AlexaFluor546 fluorochrome kits were from Invitrogen and labelling carried out as described previously (Smith et al, 2005).
The peptides were synthesized by Protein and Peptide Chemistry, Cancer Research UK, and were a 19-mer (HLSDLREYRRFEKEKLKSC) comprising the β2 subunit cytoplasmic sequence containing the α-actinin-1-binding site (β2-actinin peptide), as well as control scrambled 19-mer peptide (control peptide; KYEHRFELRLKSELDSKRC) (Pavalko and LaRoche, 1993). The peptides were linked via a disulfide bridge to the 17-mer penetratin-1 domain of antennapedia (RQIKIWFPNRRMKWKKC). They were preincubated with cells at 50 μg/ml for 30 min at 37°C.
Cells and cell transfections
Human T lymphoblasts (T cells) were prepared as described previously (Smith et al, 2003). Primary T cells were isolated from a single donor by fractionation of whole blood using Lymphoprep, and isolated by negative depletion using the human pan T cell isolation kit II (Miltenyi Biotec Ltd, Bisley, UK). The HSB-2 T cell line (Wright et al, 1994) was maintained in RPMI 1640/10% FCS. HUVECs (passage 2) were grown in Bulletkit medium (Cambrex Bio Science Wokingham Ltd, Wokingham, UK).
Before transfection, HSB-2 cells were washed twice in OptiMEM+ GlutaMAX (Invitrogen). Electroporation (4 × 107 cells/ml) was carried out with 10 μg per reaction of GFP-linked α-actinin-1 mutant missing the actin-binding site (ΔN-actinin), GFP-linked full-length human α-actinin-1 (Guvakova et al, 2002) or 5 μg of pEGFP-N1 (BD Biosciences, Oxford, UK). Alternatively, 2 × 107HSB-2 cells were transfected with 50–400 nM of siRNAs against α-actinin-1 (ID 9416 or ID 147017 or ID 16804), talin (ID 5552) or a control (ID 4611G) (Ambion Europe Ltd, Huntingdon, UK) as described previously (Smith et al, 2005). The siRNA- and DNA-transfected cells were maintained in RPMI 1640/10% FCS for 48 h and 4–24 h, respectively, before use. Efficiency of knockdowns was assessed by western blot and quantified using NIH image 1.63 software.
Confocal microscopy
T cells or HSB-2 cells (2 × 105) in HBSS were observed migrating on ICAM-1Fc-coated coverslips or on HUVECs for 10–20 min, and prestimulated with 5 ng/ml TNF-α for 16 h as described previously (Smith et al, 2003). Primary T lymphocytes were stimulated with 40–80 ng/ml of SDF-1 (Peprotech, London, UK) to induce migration. Alexa546- or 488-labelled KIM127 or 24 (5 μg/ml) was added to migrating T cells and confocal images collected every 15 s at coverslip level with a Zeiss Axiovert 100M LSM-510-META inverted microscope. Alternatively Alexa-labelled KIM127 or 24 was added to migrating cells for 2–5 min either during fixation with 4% formaldehyde or following fixation and permeabilization with 0.1% Triton X-100. Confocal images were taken using a Zeiss Axiovert LSM-510-META inverted microscope and the localization determined using LSM-510 software (Zeiss, Germany).
Video microscopy
Glass-bottomed microwell dishes (measuring 35 mm) (MatTek Corp., MA) were coated overnight with 3 μg/ml ICAM-1Fc. T cells or HSB-2 cells (4 × 105/ml in HBSS/20 mM HEPES buffer) were allowed to settle for 10 min at 37°C and then non-attached cells were removed with gentle washing. MAbs were then added and images taken with a Nikon Diaphot 300 microscope, using a × 40 or × 63 lens, and AQM2001 Kinetic Acquisition Manager software (Kinetic Imaging Ltd, UK). Cells were tracked at 5-s intervals using Motion Analysis software (Kinetic Imaging Ltd) and the data analyzed using a Mathematica notebook (Wolfram Research Inc., USA). Statistical analysis was carried out using the ANOVA test. Quantification of bound KIM127 and 24 mAbs was assessed using LSM510 software (Zeiss, Germany)
Interference reflection microscopy
T cells were pretreated with 50 μg/ml of β2-actinin or control peptide for 30 min before plating on MatTek dishes coated with 3 μg/ml ICAM-1. Alternatively, T cells were treated while migrating with 10 μg/ml mAbs KIM127 or 24. In some experiments 50 μg/ml of the β2-actinin or control peptide was added after treatment with KIM127. Images of close substrate contact of migrating T cells were acquired with the Invert Confocal microscope (as above) with a × 63 NA 1.4 Plan-Apochromat oil-immersion objective lens. Using IRM configuration with 488-nm wavelength illumination, images were recorded every 6 s. For evaluation of lamella/lamellipodial extension status, individual T cells were tracked for ∼30 s (n=40 cells per sample type). Images were scored by two observers for (i) a normal lamella displaying lamellipodial protrusions in advance of the leading edge, (ii) a single limited lamellar protrusion or (iii) lack of a formal lamella.
Immunoprecipitation and western blotting
T cells (5 × 107) were incubated with 10 μg/ml KIM127, 24, 38 or control mAb, 52 U, in HBSS/20 mM HEPES buffer+2.5 mM MgCl2 for 20 min at 37°C, and then washed to remove unbound antibody. The cells were lysed with 1 ml ice-cold buffer (1% Triton X-100, 50 mM Tris base pH 8.0, 150 mM NaCl, 2 mM MgCl2, 2 mM EGTA, complete protease inhibitor cocktail; Roche Diagnostics Ltd, Lewes, UK) and 0.1 mM PMSF. The T cell lysate was incubated with 50% each of sepharose-linked protein G and A (GE Healthcare UK Ltd., Chalfont St Giles, UK) for 5 h at 4°C before SDS–PAGE separation. A 10-μl volume of whole T cell lysate was used as a positive control. Nitrocellulose blots were probed with mAbs for α-actinin-1 and αL. Bound mAb was detected with sheep anti-mouse IgG–HRP and ECL detection reagents (GE Healthcare). For α-actinin-1 knockdown experiments, total cell lysates were blotted and detected with α-actinin-1, talin or α-tubulin Abs.
To calculate the proportion of total α-actinin-1 bound to LFA-1, the density of the band of α-actinin-1 co-immunoprecipitated by a particular anti-LFA-1 mAb from 1 ml of lysate was compared with the density of the band from total α-actinin-1 in the T cell lysate × volume of sample. The immunoprecipitations were carried out at 4°C to minimize differences in mAb affinity. The calculations represent averages of three experiments.
Pull-down assay
T cells (5 × 107) were lysed with ice-cold buffer (1% NP-40, 20 mM Tris base pH 7.4, 150 mM NaCl, 5 mM MgCl2, complete protease inhibitor cocktail, 0.1 mM PMSF and 250 U benzonase). Biotin-labelled β2-actinin peptide or control peptide (50 μg/ml) was incubated at 4°C for 2 h. Streptavidin microbeads (Miltenyi Biotec Ltd, Bisley, UK) were added for 1 h at 4°C, passed through an MACS separation column and washed with 10 ml lysis buffer. Bound beads were boiled in reducing SDS–PAGE sample buffer. Western blot analysis was performed with mAbs for α-actinin-1, talin and vinculin as above.
Cell adhesion assays
Flat-well Immulon 1B 96-well plates were coated with 3 μg/ml ICAM-1Fc as described previously (Smith et al, 2005). T cells or HSB-2 cells were labelled with 2.5 μM 2′,7′-bis-(carboxyethyl)-5(6′)-carboxyfluorescein (BCECF; Merck Biosciences Ltd.), washed in HBSS/20 mM HEPES, plated at 2 × 105/well and incubated at 37°C for 30 min. Non-adherent cells were removed by gentle washing and adhesion quantified using a Cytofluor multiwell plate reader (PerSeptive Biosystems, Hertford, UK).
Transwell chemotaxis assay
For migration assays, 107/ml T cells in RPMI/0.5% BSA with or without 10 μg/ml mAb were added to the upper well of Transwell chambers (6.5 mm diameter, 5 μm pores; Corning Costar) coated for 16 h at 4°C with 3 μg/ml ICAM-1Fc. Cells migrated at 37°C toward 600 μl of 10 nM SDF-1 (Peprotech EC Ltd., London, UK) in the lower well. After 1 h cells in the lower well were quantified.
Acceptor depletion FRET
HSB2 T cells that were stably transfected with LFA-1 bearing GFP-tagged β2 subunit (Smith et al, 2005) were allowed to migrate on immobilized ICAM-1 for 30 min before fixation with 3% HCHO, followed by labelling with Alexa 546-conjugated anti-α-actinin mAb. In some experiments, the cells were pretreated for 20 min with β2-actinin or scrambled control peptide (50 μg/ml) before adding to the ICAM-1 coated plates for 30 min. AdFRET was carried out as described in Di et al (2005).
Supplementary Material
Supplementary movie 1
Supplementary movie 2
Supplementary movie 3
Supplementary movie 4
Supplementary movie 5
Supplementary movie 6
Supplementary movie 7
Supplementary movie 8
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Data
Acknowledgments
We thank Drs M Robinson (Celltech Group, UK) and M Guvakova (University of Pennsylvania, USA) for gifts of mAb KIM127 and α-actinin–GFP constructs, respectively. We are grateful to CR UK LRI and Leukocyte Adhesion Laboratory colleagues for discussion and comments. We thank Dr Tatsuo Kinashi, Kansai Medical University, Osaka, Japan for permission to mention his unpublished data. This work was supported by Cancer Research UK.
References
- Alonso JL, Essafi M, Xiong JP, Stehle T, Arnaout MA (2002) Does the integrin alphaA domain act as a ligand for its betaA domain? Curr Biol 12: R340–R342 [DOI] [PubMed] [Google Scholar]
- Beglova N, Blacklow SC, Takagi J, Springer TA (2002) Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nat Struct Biol 9: 282–287 [DOI] [PubMed] [Google Scholar]
- Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL (2001) Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol 153: 881–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman CV, Springer TA (2003) Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol 15: 547–556 [DOI] [PubMed] [Google Scholar]
- Cyster JG (2005) Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol 23: 127–159 [DOI] [PubMed] [Google Scholar]
- de Pereda JM, Wegener KL, Santelli E, Bate N, Ginsberg MH, Critchley DR, Campbell ID, Liddington RC (2005) Structural basis for phosphatidylinositol phosphate kinase type Igamma binding to talin at focal adhesions. J Biol Chem 280: 8381–8386 [DOI] [PubMed] [Google Scholar]
- Di WL, Gu Y, Common JE, Aasen T, O'Toole EA, Kelsell DP, Zicha D (2005) Connexin interaction patterns in keratinocytes revealed morphologically and by FRET analysis. J Cell Sci 118: 1505–1514 [DOI] [PubMed] [Google Scholar]
- Dransfield I, Cabanas C, Barrett J, Hogg N (1992a) Interaction of leukocyte integrins with ligand is necessary but not sufficient for function. J Cell Biol 116: 1527–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield I, Cabanas C, Craig A, Hogg N (1992b) Divalent cation regulation of the function of the leukocyte integrin LFA-1. J Cell Biol 116: 219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Bivona TG, Philips MR (2004) Membranes as messengers in T cell adhesion signaling. Nat Immunol 5: 363–372 [DOI] [PubMed] [Google Scholar]
- Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER (1997) Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci USA 94: 3909–3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M, Di Meglio S, Gagliani MC, Consonni E, Molteni R, Bender JR, Tacchetti C, Pardi R (2005) Dynamic partitioning into lipid rafts controls the endo-exocytic cycle of the alphaL/beta2 integrin, LFA-1, during leukocyte chemotaxis. Mol Biol Cell 16: 5793–5803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guvakova MA, Adams JC, Boettiger D (2002) Functional role of alpha-actinin, PI 3-kinase and MEK1/2 in insulin-like growth factor I receptor kinase regulated motility of human breast carcinoma cells. J Cell Sci 115: 4149–4165 [DOI] [PubMed] [Google Scholar]
- Hogg N, Henderson R, Leitinger B, McDowall A, Porter J, Stanley P (2002) Mechanisms contributing to the activity of integrins on leukocytes. Immunol Rev 186: 164–171 [DOI] [PubMed] [Google Scholar]
- Hogg N, Laschinger M, Giles K, McDowall A (2003) T-cell integrins: more than just sticking points. J Cell Sci 116: 4695–4705 [DOI] [PubMed] [Google Scholar]
- Kamata T, Tieu KK, Tarui T, Puzon-McLaughlin W, Hogg N, Takada Y (2002) The role of the CPNKEKEC sequence in the beta(2) subunit I domain in regulation of integrin alpha(L)beta(2) (LFA-1). J Immunol 168: 2296–2301 [DOI] [PubMed] [Google Scholar]
- Kelly DF, Taylor DW, Bakolitsa C, Bobkov AA, Bankston L, Liddington RC, Taylor KA (2006) Structure of the alpha-actinin–vinculin head domain complex determined by cryo-electron microscopy. J Mol Biol 357: 562–573 [DOI] [PubMed] [Google Scholar]
- Kelly DF, Taylor KA (2005) Identification of the beta1-integrin binding site on alpha-actinin by cryoelectron microscopy. J Struct Biol 149: 290–302 [DOI] [PubMed] [Google Scholar]
- Kinashi T (2005) Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol 5: 546–559 [DOI] [PubMed] [Google Scholar]
- Kinashi T (2006) Adhere upright: a switchblade-like extension of beta2 integrins. Immunity 25: 521–522 [DOI] [PubMed] [Google Scholar]
- Kiosses WB, Shattil SJ, Pampori N, Schwartz MA (2001) Rac recruits high-affinity integrin alphavbeta3 to lamellipodia in endothelial cell migration. Nat Cell Biol 3: 316–320 [DOI] [PubMed] [Google Scholar]
- Laukaitis CM, Webb DJ, Donais K, Horwitz AF (2001) Differential dynamics of alpha 5 integrin, paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol 153: 1427–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Shimaoka M, Zang Q, Takagi J, Springer TA (2001) Locking in alternate conformations of the integrin alphaLbeta2 I domain with disulfide bonds reveals functional relationships among integrin domains. Proc Natl Acad Sci USA 98: 2393–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo BH, Carman CV, Springer TA (2007) Structural basis of integrin regulation and signaling. Annu Rev Immunol 25: 619–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mempel TR, Henrickson SE, Von Andrian UH (2004) T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427: 154–159 [DOI] [PubMed] [Google Scholar]
- Nishida N, Xie C, Shimaoka M, Cheng Y, Walz T, Springer TA (2006) Activation of leukocyte beta2 integrins by conversion from bent to extended conformations. Immunity 25: 583–594 [DOI] [PubMed] [Google Scholar]
- Otey CA, Carpen O (2004) Alpha-actinin revisited: a fresh look at an old player. Cell Motil Cytoskeleton 58: 104–111 [DOI] [PubMed] [Google Scholar]
- Pavalko FM, LaRoche SM (1993) Activation of human neutrophils induces an interaction between the integrin beta 2-subunit (CD18) and the actin binding protein alpha-actinin. J Immunol 151: 3795–3807 [PubMed] [Google Scholar]
- Porter JC, Bracke M, Smith A, Davies D, Hogg N (2002) Signaling through integrin LFA-1 leads to filamentous actin polymerization and remodeling, resulting in enhanced T cell adhesion. J Immunol 168: 6330–6335 [DOI] [PubMed] [Google Scholar]
- Robinson MK, Andrew D, Rosen H, Brown D, Ortlepp S, Stephens P, Butcher EC (1992) Antibody against the Leu-CAM beta-chain (CD18) promotes both LFA-1- and CR3-dependent adhesion events. J Immunol 148: 1080–1085 [PubMed] [Google Scholar]
- Sampath R, Gallagher PJ, Pavalko FM (1998) Cytoskeletal interactions with the leukocyte integrin beta2 cytoplasmic tail. Activation-dependent regulation of associations with talin and alpha-actinin. J Biol Chem 273: 33588–33594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmrich M, Smith A, Feterowski C, Beer S, Engelhardt B, Busch DH, Bartsch B, Laschinger M, Hogg N, Pfeffer K, Holzmann B (2005) Importance of integrin LFA-1 deactivation for the generation of immune responses. J Exp Med 201: 1987–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SK, Ma S, Kim MB, Rao RM, Hartman CU, Froio RM, Yang L, Jones T, Liu Y, Nusrat A, Parkos CA, Luscinskas FW (2004) Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J Exp Med 200: 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Bracke M, Leitinger B, Porter JC, Hogg N (2003) LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J Cell Sci 116: 3123–3133 [DOI] [PubMed] [Google Scholar]
- Smith A, Carrasco YR, Stanley P, Kieffer N, Batista FD, Hogg N (2005) A talin-dependent LFA-1 focal zone is formed by rapidly migrating T lymphocytes. J Cell Biol 170: 141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi J, Petre BM, Walz T, Springer TA (2002) Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110: 599–611 [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mempel TR (2003) Homing and cellular traffic in lymph nodes. Nat Rev Immunol 3: 867–878 [DOI] [PubMed] [Google Scholar]
- von Wichert G, Haimovich B, Feng GS, Sheetz MP (2003) Force-dependent integrin–cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. EMBO J 22: 5023–5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID (2007) Structural basis of integrin activation by talin. Cell 128: 171–182 [DOI] [PubMed] [Google Scholar]
- Wiseman PW, Brown CM, Webb DJ, Hebert B, Johnson NL, Squier JA, Ellisman MH, Horwitz AF (2004) Spatial mapping of integrin interactions and dynamics during cell migration by image correlation microscopy. J Cell Sci 117: 5521–5534 [DOI] [PubMed] [Google Scholar]
- Wright DD, Sefton BM, Kamps MP (1994) Oncogenic activation of the Lck protein accompanies translocation of the LCK gene in the human HSB2 T-cell leukemia. Mol Cell Biol 14: 2429–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA (2001) Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science 294: 339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary movie 1
Supplementary movie 2
Supplementary movie 3
Supplementary movie 4
Supplementary movie 5
Supplementary movie 6
Supplementary movie 7
Supplementary movie 8
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Data