Abstract
A large number of trafficking steps occur between the last compartment of the Golgi apparatus (TGN) and the vacuole of the yeast Saccharomyces cerevisiae. To date, two intracellular routes from the TGN to the vacuole have been identified. Carboxypeptidase Y (CPY) travels through a prevacuolar/endosomal compartment (PVC), and subsequently on to the vacuole, while alkaline phosphatase (ALP) bypasses this compartment to reach the same organelle. Proteins resident to the TGN achieve their localization despite a continuous flux of traffic by continually being retrieved from the distal PVC by virtue of an aromatic amino acid–containing sorting motif. In this study we report that a hybrid protein based on ALP and containing this retrieval motif reaches the PVC not by following the CPY sorting pathway, but instead by signal-dependent retrograde transport from the vacuole, an organelle previously thought of as a terminal compartment. In addition, we show that a mutation in VAC7, a gene previously identified as being required for vacuolar inheritance, blocks this trafficking step. Finally we show that Vti1p, a v-SNARE required for the delivery of both CPY and ALP to the vacuole, uses retrograde transport out of the vacuole as part of its normal cellular itinerary.
Keywords: endosome, SNARE, TGN, vacuole, VPS
Proteins traveling to the lysosome of mammalian cells and the analogous vacuole in yeast cells reach their destination by following one of many pathways (Griffiths et al., 1988; Ludwig et al., 1991; Rabouille et al., 1993; Hunziker and Geuze, 1996; Liou et al., 1997; Bryant and Stevens, 1998). With the exception of resident lysosomal/vacuolar proteins, which tend to be degradative enzymes, most of these proteins are ultimately degraded and as such the lysosome and vacuole are viewed as the terminal compartments of the endosomal system (DeDuve, 1963; Kornfeld and Mellman, 1989). The endosomal system is clearly a very dynamic system with many proteins cycling between various compartments within it as part of their normal cellular itinerary (Kornfeld and Mellman, 1989; Nothwehr and Stevens, 1994). To date, recycling of proteins is thought to occur from compartments proximal to the lysosome/vacuole, and the possibility that proteins can recycle out of these hydrolytic compartments has been largely overlooked because of the difficulty of measuring such movement. Recent studies, however, indicate that lysosomes are far more dynamic than was previously appreciated, and that they are in close communication with endocytic compartments perhaps making retrograde traffic from the lysosome more likely (Hunziker and Geuze, 1996; Storrie and Desjardins, 1996). These studies indicate that transport to the lysosome involves the fusion of late endosomes with pre-existing lysosomes (van Deurs et al., 1995; Futter et al., 1996; Storrie and Desjardins, 1996; Bright et al., 1997; Mullock et al., 1998), or possibly involves a distinct class of transport vesicles (Berg et al., 1995). Either scenario invokes the need to reacquire at least the specific transport proteins, such as putative SNAP receptor (SNARE)1 proteins, that may mediate such a process.
Studies on the trafficking of lysosomal membrane proteins in the presence of cycloheximide, wortmanin, or the vacuolating toxin, VacA, indicate that proteins such as Lamp-1 continually cycle between lysosomes and late endosomes (Lippincott-Schwartz and Fambrough, 1987; Akasaki et al., 1993, 1995; Reaves et al., 1996). Using a different model system, Brachet et al. (1997) showed protein traffic out of the lysosome-like MHC class II compartment is possible for MHC class II a/b chain complexes when degradation of the invariant chain is blocked. Other experiments indicate that soluble lysosomal constituents may also participate in retrograde transport (Jahrous et al., 1994). A mechanism for how proteins could be retrieved from the lysosome is provided by the observation that AP-2/clathrin coats can assemble specifically on the surface of lysosomes thus providing a specific mechanism for the budding of transport intermediates (i.e., vesicles) from the lysosome (Traub et al., 1996). While these above studies provide support for the occurrence of retrograde transport from the lysosome, they are complicated by the use of pharmacological experimental manipulations that may compromise the distinction and function of late endosomes and lysosomes. Furthermore, it has not been possible to find a particular protein that clearly follows such a pathway under normal conditions.
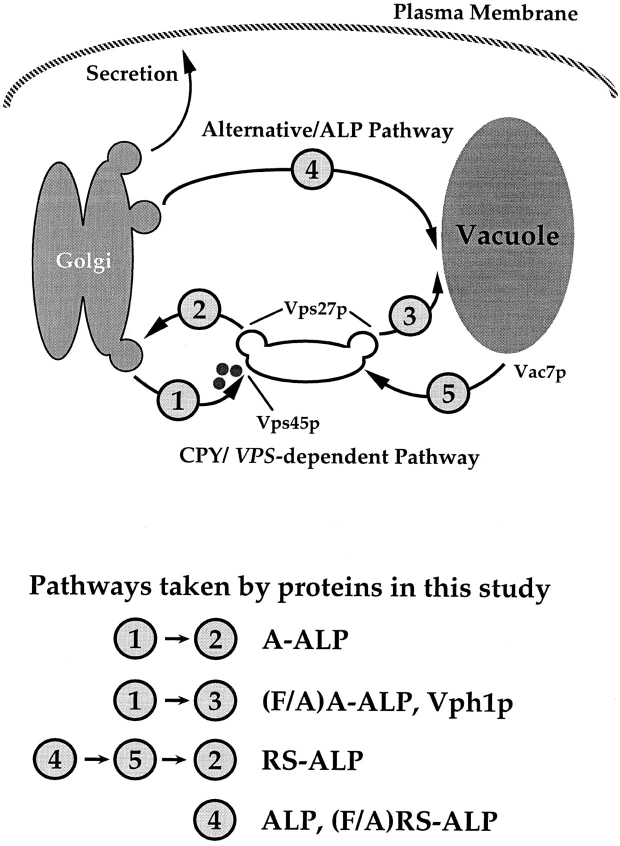
Vacuolar biogenesis in Saccharomyces cerevisiae has very strong parallels with lysosomal biogenesis in mammalian cells (Stack et al., 1995; Bryant and Stevens, 1998). The delivery of vacuolar hydrolases such as carboxypeptidase Y (CPY) relies on the cycling of the CPY receptor, Vps10p, between the TGN and the prevacuolar/endosomal compartment (PVC) in a manner analogous to the delivery of modified hydrolases by the mannose-6-phosphate receptor (Stack et al., 1995). In this pathway the fusion of Golgi-derived vesicles that contain Vps10p with the PVC is mediated by the Sec1p-like protein, Vps45p, the target (t)-SNARE protein Pep12p, the Rab protein, Vps21p/ Ypt51, and the vesicle (v)-SNARE protein Vti1p (Cowles et al., 1994; Horazdovsky et al., 1994; Piper et al., 1994; Singer-Kruger et al., 1994; Becherer et al., 1996; Fischer von Mollard et al., 1997). Efflux of traffic from the PVC is controlled by class E Vps proteins such as Vps27p and Vps4p and these proteins may function by creating the necessary transport intermediates that ultimately fuse with the vacuole (Piper et al., 1995; Babst et al., 1997). Fusion to the vacuole itself is controlled in part by the vacuolar t-SNARE Vam3p, although it is unclear whether final delivery of CPY from the PVC requires a vesicular carrier or the fusion of a larger endosomal compartment with the vacuole as has been proposed for the delivery to lysosomes (Futter et al., 1996; Storrie and Desjardins, 1996; Bright et al., 1997). A second intracellular route to the vacuole from the TGN is taken by the membrane proteins alkaline phosphatase (ALP) and Vam3p (Cowles et al., 1997; Piper et al., 1997). These proteins reach the vacuole by a transport pathway that is independent of Vps45p, Pep12p, and Vps27p function implying that they do not enter into Golgi-derived vesicles that fuse with the PVC (Piper et al., 1997). Transport of ALP to the vacuole does require Vam3p and the adaptor complex AP-3 as well as the dynamin homologue Vps1p indicating that ALP may be specifically sorted into a separate class of transport vesicles that rely on the vacuolar t-SNARE Vam3p for fusion to the vacuole (Nothwehr et al., 1995; Cowles et al., 1997; Stepp et al., 1997). This pathway appears to parallel a similar pathway in mammalian cells where a subset of lysosomal proteins such as LIMP-II and tyrosinase may be sorted by AP-3 in the TGN for ultimate delivery to the lysosome (Honing et al., 1998).
Previous studies have shown that the cytosolic tail of ALP contains sorting information sufficient for direction into the alternative pathway (Cowles et al., 1997; Piper et al., 1997). Our laboratory has previously reported that the motif FXFXD identified within the cytosolic tail of the resident TGN protein dipeptidyl aminopeptidase (DPAP) A mediates retrieval from the PVC back to the TGN (Nothwehr et al., 1993; Bryant and Stevens, 1997). Transplantation of this motif into the cytosolic tail of ALP results in a protein, RS-ALP (retention sequence-ALP), which achieves steady state localization to the TGN through continual retrieval from a post-Golgi compartment. In RS-ALP, the FXFXD retrieval motif is adjacent to a sorting domain for the alternative pathway. In this present study we report that like the wild-type ALP protein, RS-ALP follows the alternative route to the vacuole in a manner that was independent of Vps45p and Vps27p function. Furthermore, this protein was efficiently retrieved from the vacuole to the TGN via the PVC. We have identified vac7 mutants as being defective in this retrograde trafficking pathway out of the vacuole and also found that the v-SNARE protein Vti1p uses this pathway to cycle out of the vacuole as part of its normal cellular itinerary.
Materials and Methods
Materials
Enzymes used in DNA manipulations were purchased from New England Biolabs Inc. (Beverly, MA), Boehringer Mannheim Corp. (Indianapolis, IN), Bethesda Research Laboratories (Gaithersburg, MD), or United States Biochemical Corp. (Cleveland, OH). Secondary antibodies used for indirect immunofluorescence (all cross-species absorbed) were purchased from Jackson Immunoresearch Laboratories Inc. (West Grove, PA). mAbs specific for ALP (1D3-A10) are available from Molecular Probes, Inc. (Eugene, OR). Fixed Staphylococcus aureus cells (Ig sorb) were obtained from The Enzyme Center (Malden, MA). [35S]Express label was from New England Nuclear (Boston, MA). Oxalyticase was from Enzogenetics (Corvallis, OR). All other reagents were purchased from Sigma Chemical Co. (St. Louis, MO).
Strains, Media, and Microbiological Techniques
Yeast strains used in this study are listed in Table I. Strains were constructed by standard genetic techniques and grown in rich media (1% yeast extract, 1% peptone, 2% dextrose; YEPD) or standard minimal medium with appropriate supplements (Sherman et al., 1986). Strain NBY88 was derived from RPY2 (Piper et al., 1995) by transforming with pSN111 (pho8Δ-X construct) linearized with SalI (Nothwehr et al., 1995). Ura+ transformants were plated onto media containing 5-FOA to select for Ura− loopouts and pho8Δ-X colonies were identified through immunoblot analysis. NBY72, NBY86, NBY73, and NBY89 were similarly derived from SF838-9D (Rothman et al., 1989), RHY6210 (Gomes de Mesquita et al., 1996), and RPY3 (Piper et al., 1995) and LWY2809, respectively. LWY2809 is a sister spore of LWY2806 (Bonangelino et al., 1997). (NBY85 was derived from NBY89 by transforming cells with EcoRI-linearized pLO2010 (Nothwehr et al., 1995). Ura+ transformants were plated onto media containing 5-FOA and pep4Δ-X colonies were identified using the APNE plate assay (Wolf and Fink, 1975). NBY100 was made by using pSN111 to delete PHO8 in RPY1017. RPY1017 was made by transforming SEY6210 to His+ using a PCR product encompassing the HIS3 gene flanked by 49–100 and 1,351–1,402 relative to the start codon. This HIS3 disruption resulted in the deletion of the region corresponding to amino acids 33–450 of the APM3 gene was confirmed by PCR analysis of the resulting APM3 locus.
Table I.
Yeast Strains Used in This Study
| Strain | Genotype | Source | ||
|---|---|---|---|---|
| AACY28 | MATα ura3-52 leu2-3,112 his4-519 ade6 pho8Δ::LEU2 | Cooper and Stevens, 1996 | ||
| NBY68 | MATα ura3-52 leu2-3,112 his4-519 ade6 vps45Δ-X pho8Δ-X | Piper et al., 1997 | ||
| NBY88 | MATα ura3-52 leu2-3,112 his4-519 ade6 vps27Δ::LEU2 pho8Δ-X | This study | ||
| NBY72 | MATα ura3-52 leu2-3,112 his4-519 ade6 pho8-ΔX pep4-3 | This study | ||
| NBY83 | MATα ura3-52 leu2-3,112 his4-519 ade6 vps45Δ-X pho8Δ-X pep4Δ-X | Bryant et al., 1998 | ||
| NBY60 | MATα ura3-52 leu2-3,112 his4-519 ade6 vps27Δ::LEU2 pho8Δ-X pep4-3 | Bryant et al., 1998 | ||
| NBY84 | MATα ura3-52 leu2-3,112 his4-519 ade6 vps27Δ::LEU2 vps45Δ-X pho8-ΔX pep4-3 | Bryant et al., 1998 | ||
| RPY103 | MATα ura3-52 leu2-3,112 his4-519 ade6 vps27-ts pep4-3 PS10::LEU2::vps10-10* | Piper et al., 1997 | ||
| NBY73 | MATα ura3-52 leu2-3,112 his4-519 ade6 vps27-ts pho8Δ-X pep4-3 | This study | ||
| NBY86 | MATα ura3-52 leu2-3,112 his3-Δ200 trp1-901 lys2-801 suc2-Δ9 pho8Δ-X pep4-Δ1137 | This study | ||
| SEY6210 | MATα ura3-52 leu2-3,112 his3-Δ200 trp1-901 lys2-801 suc2-Δ9 | Robinson et al., 1988 | ||
| LWY2809 | MAT a ura3-52 leu2-3,112 his3-Δ200 trp1-Δ901 lys2-801 ade28::HIS3 suc2-Δ9 vac7-1 | This study | ||
| NBY85 | MAT a ura3-52 leu2-3,112 his3-Δ200 trp1-Δ901 lys2-801 ade8::HIS3 suc2-Δ9 vac7-1 pho8Δ-X pep4Δ-X | This study | ||
| NBY89 | MAT a ura3-52 leu2-3,112 his3-Δ200 trp1-Δ901 lys2-801 ade8::HIS3 suc2-Δ9 vac7-1 pho8Δ-X | This study | ||
| SEY6210 vpt13 | MATα ura3-52 leu2-3,112 his3-Δ200 trp1-901 lys2-801 suc2-Δ9 vpt13 | Robinson et al., 1988 | ||
| NBY100 | MATα ura3-52 leu2-3,112 his3-Δ200 trp1-901 lys2-801 suc2-Δ9 pho8-ΔX apm3::HIS3 | This study |
Plasmid Construction
Plasmids used in this study are listed in Table II. DNA manipulations and DNA-mediated transformation of E. coli strains CJ236 and XL-1 blues were performed by routine procedures (Sambrook et al., 1989). pSN123, a CEN-URA3 plasmid encoding (F/A)RS-ALP was constructed by oligonucleotide directed mutagenesis of pSN97 according to the method of Kunkel et al. (1987). The resulting plasmid encodes a version of RS-ALP in which the two phenylalanine residues contained within the Golgi localization motif (RRESFQFNDI) transplanted from Ste13p into the cytosolic tail of ALP (Nothwehr et al., 1993) have been mutated to alanine residues. pNB7, a CEN-LEU2 plasmid encoding RS-ALP, was constructed by subcloning the 4-kb BamHI fragment from pSN97 into the BamHI site of pRS315 (Sikorski and Hieter, 1989). pNB8, a CEN-LEU2 plasmid encoding (F/A)A-ALP, was constructed by subcloning the SacI–EcoRV fragment encompassing the STE13-PHO8 gene fusion encoding (F/A)A-ALP from pSN100 into the SacI–SmaI sites of pRS315 (Sikorski and Hieter, 1989).
Table II.
Plasmids Used in This Study
| Plasmid | Description | Source | ||
|---|---|---|---|---|
| pSN92 | CEN-URA3 plasmid endoding ALP | Nothwehr et al., 1993 | ||
| pSN97 | CEN-URA3 plasmid encoding RS-ALP | Nothwehr et al., 1993 | ||
| pSN123 | CEN-URA3 plasmid encoding (F/A)RS-ALP | This study | ||
| pSN55 | CEN-URA3 plasmid encoding A-ALP | Nothwehr et al., 1993 | ||
| pSN100 | CEN-URA3 plasmid encoding (F/A)A-ALP | Nothwehr et al., 1993 | ||
| pNB7 | CEN-LEU2 plasmid encoding RS-ALP | This study | ||
| pNB8 | CEN-LEU2 plasmid encoding (F/A)A-ALP | This study | ||
| pSN111 | integrating plasmid for pho8-ΔX allele (loop in/loop out) | Nothwehr et al., 1995 | ||
| pLO2010 | integrating plasmid for pep4-ΔH3 allele (loop in/loop out) | Nothwehr et al., 1995 | ||
| pTS63 | CEN-LEU2 plasmid encoding Vps10p-Δ10* | Cooper and Stevens, 1996 | ||
| pRCP39 | GAL-PEP4 in CEN-URA plasmid | Piper et al., 1997 |
Radiolabeling and Immunoprecipitation
[35S]Methionine labeling and immunoprecipitation of ALP fusion proteins, Vps10p-related proteins, and CPY were performed as previously described (Piper et al., 1994; Nothwehr et al., 1995; Cooper and Stevens, 1996). In brief, yeast cultures were grown overnight in selective synthetic media without methionine to OD600 = 1. Cells were harvested and resuspended in fresh media to the same OD600. Cells were pulse labeled for 10 min with 100 μCi 35S-Express label per 0.5 OD600, followed by the addition of unlabeled methionine and cysteine both to 50 μg/ml. At specified times samples were removed and treated by the addition of sodium azide to 10 mM at 4°C. Vps10p, CPY, ALP, and related proteins were quantitatively immunoprecipitated from protein extracts of these cells using the relevant specific polyclonal antibodies as previously described (Piper et al., 1994; Nothwehr et al., 1995; Cooper and Stevens, 1996). Half-times of processing of ALP- and Vps10p-related proteins were determined as previously described (Nothwehr et al., 1993; Cooper and Stevens, 1996) using an AMBIS Radioanalytic Imaging System (Ambis, San Diego, CA) and linear regression analysis plotting percentage total protein processed as a function of time.
Immunofluorescence Microscopy
The preparation of fixed yeast cell spheroplasts, their attachment to microscope slides, and co-staining of ALP fusion proteins using the mouse anti-ALP mAb 1D3-A10 (Molecular Probes Inc.) and Vph1p using affinity-purified polyclonal antibodies was carried out as previously described (Cooper and Stevens, 1996; Piper et al., 1997). Before use, the mouse anti-ALP mAb 1D3-A10 (Molecular Probes Inc.) was adsorbed against pho8Δ yeast cells to increase the ALP-specific signal. pho8Δ yeast cells were fixed, converted to spheroplasts, and then permeabilized using 1% SDS for 1 min. These were resuspended in 1D3-A10, diluted in PBS containing 5 mg/ml BSA, and then incubated for 1 h at 25°C. Spheroplasts were removed by centrifugation and the resultant supernatant was used to stain fixed cells attached to microscope slides. Essentially, fixed spheroplasts attached to slides were incubated with the following solutions, followed by extensive washing with PBS containing 5 mg/ml BSA after each step (all antibody incubations were performed at 25°C for 1 h with the exception of those involving the mouse anti-ALP mAb 1D3-A10 cultured supernatant; these were performed at 4°C for 12–16 h): (a) PBS-BSA containing a 1:3 dilution of adsorbed 1D3-A10 cultured supernatant; (b) 1:500 dilution of a biotin-conjugated donkey anti–mouse IgG (H + L) and a 1:20 dilution of affinity-purified rabbit anti-Vph1p polyclonal antibody; and (c) 1:500 dilution of FITC-conjugated streptavidin and 1:2,000 dilution of Texas red–conjugated goat anti–rabbit IgG (H + L). Co-staining of ALP with Vti1p was performed using 1D3-A10 cultured supernatant at a dilution of 1:3 in conjunction with affinity-purified antibodies specific for Vti1p as previously described (Fischer von Mollard et al., 1997). In brief, the incubations were as follows: (a) PBS-BSA containing a 1:50 dilution of the affinity-purified polyclonal antibody, (b) followed by a 1:500 dilution of a biotin-conjugated donkey anti–rabbit IgG (H + L) and a 1:3 dilution of 1D3-A10, and (c) 1:500 dilution of FITC-conjugated streptavidin and 1:2,000 dilution of Texas red–conjugated goat anti–mouse IgG (H + L). Similarly, co-staining of RS-ALP and Vps10p was performed as previously described (Cooper and Stevens, 1996) using a 1:200 dilution of affinity-purified polyclonal antibodies that recognize Vps10p in conjunction with a 1:3 dilution of 1D3-A10. Staining for Pep12p was performed using affinity-purified rabbit anti-Pep12p antibodies (Fischer von Mollard et al., 1997) in conjunction with biotin/streptavidin amplification as described above. Images were captured on 35 mm Tmax −400 film with a 100× oil immersion lens on an Axioplan fluorescence microscope (Carl Zeiss, Oberkochen, Germany). Film negatives were digitized using a Polaroid SprintScan 35. Images were adjusted with standard settings using Photoshop™ (Adobe Systems Inc., Mountain View, CA).
Results
Ablation of the Retrieval Motif of the Model Recycling Golgi Membrane Protein, RS-ALP, Causes Mislocalization to the Vacuole
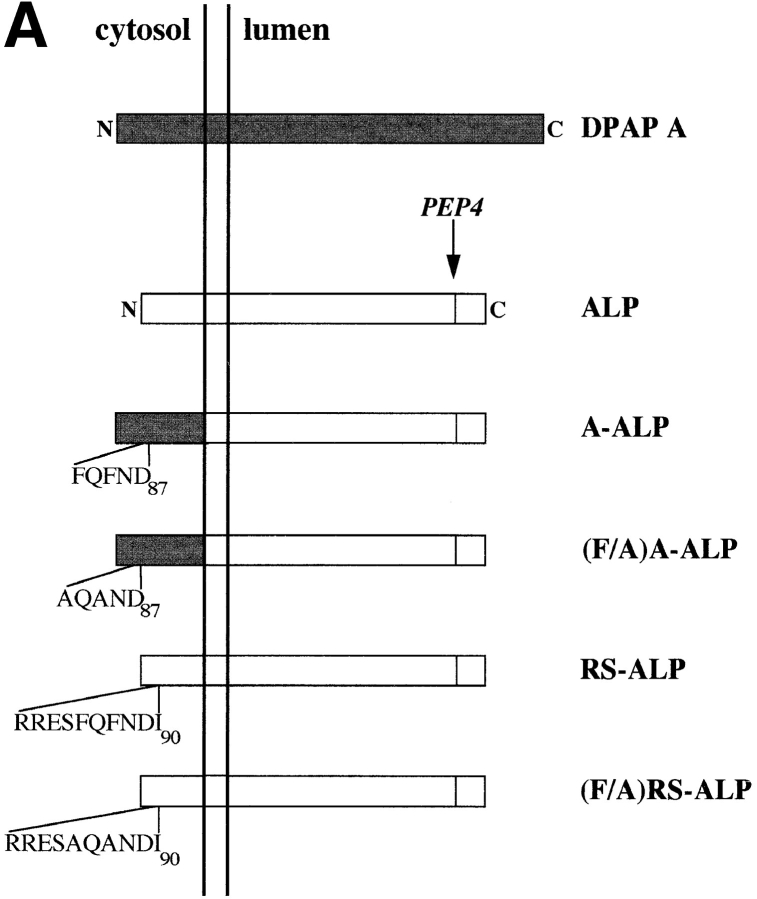
Our laboratory has previously described the protein A-ALP, which was constructed by fusing the cytosolic domain of the TGN protein DPAP A to the transmembrane and lumenal domains of the vacuolar protein ALP (Nothwehr et al., 1993). A-ALP is found in the TGN of wild-type cells, and achieves this localization through a combination of static retention mechanisms and retrieval from the post-Golgi PVC (Nothwehr et al., 1993; Bryant and Stevens, 1997). Fig. 1 shows a schematic representation of A-ALP and the other hybrid proteins used in this study, as well as their cellular localization in wild-type cells. In (F/A)A-ALP, the two phenylalanine residues contained within the FXFXD motif responsible for the retrieval of DPAP A and A-ALP from the PVC back to the Golgi have been mutated to alanine residues (Nothwehr et al., 1993). Because of this mutation, (F/A)A-ALP cannot be retrieved from the PVC to the TGN, and consequently travels on to the vacuolar membrane (Nothwehr et al., 1993; Bryant and Stevens, 1997). RS-ALP was constructed by transplanting a 10-residue stretch from DPAP A, encompassing the FXFXD motif, into the cytosolic domain of ALP (Nothwehr et al., 1993). This transplantation inserted the retrieval motif (RRESFQFNDI) in place of residues 11–17 (TRLVPGS) from the 33-residue tail of ALP, and the resulting protein localizes to the TGN of wild-type cells (Nothwehr et al., 1993). Using indirect immunofluorescence and subcellular fractionation, previous studies have shown that RS-ALP, like A-ALP, colocalizes with the TGN marker protein Kex2p (Nothwehr et al., 1993; Bryant and Stevens, 1997). Like A-ALP, RS-ALP achieves its localization by continuous retrieval from a post-Golgi compartment but does not use the static retention mechanism(s) used by DPAP A and A-ALP (Bryant and Stevens, 1997).
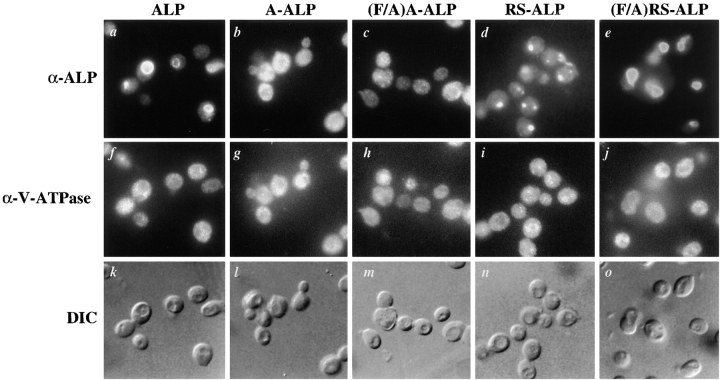
Figure 1.
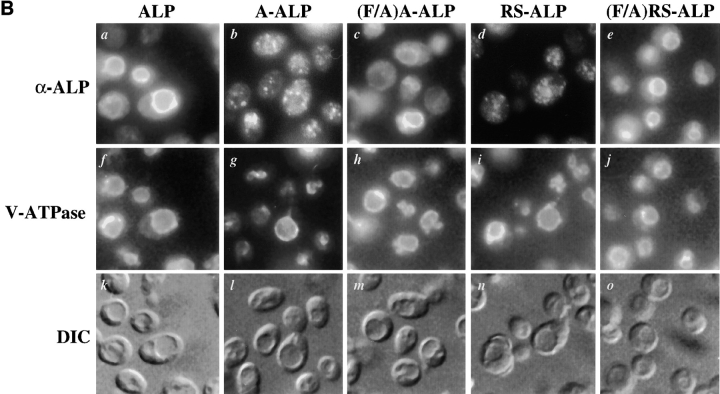
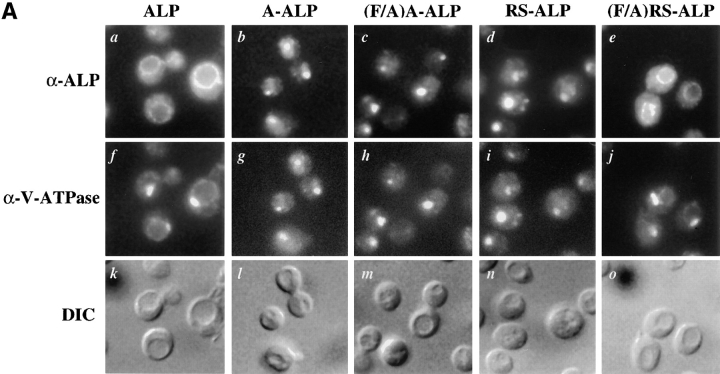
(A) Schematic representation of proteins used in this study. Sequences derived from DPAP A are shown shaded and those from ALP are unshaded. (B) Localization of ALP, A-ALP, (F/A)A-ALP, RS-ALP, and (F/A)RS-ALP in wild-type cells. NBY72 (pho8Δ-X pep4-3) cells harboring pSN92 (ALP; a, f, and k), pSN55 (A-ALP; b, g, and l), pSN100 ((F/A)A-ALP; c, h, and m), pSN97 (RS-ALP; d, i, and n), or pSN123 ((F/A)RS-ALP; e, j, and o) were prepared for double labeling indirect immunofluorescence using the α-ALP mAb, 1D3-A10 (a–e) and affinity-purified antibodies against the 100-kD subunit of the V-ATPase, Vph1p, to show the localization pattern of the V-ATPase (f–j) as described in Materials and Methods. Cells were also visualized using DIC microscopy (k–o).
Fig. 1 B shows double-labeling immunofluorescence for the 100-kD subunit of the vacuolar ATPase (V-ATPase), Vph1p, and the various ALP-based proteins depicted in Fig. 1 A. Whereas ALP was found on the vacuolar membrane of wild-type cells (Fig. 1 B, a), both A-ALP and RS-ALP localized to punctate structures (Fig. 1 B, b and d) characteristic of markers of the TGN (Franzusoff et al., 1991; Redding et al., 1991; Nothwehr et al., 1993). This localization requires the presence of the two phenylalanine residues within the FXFXD motif, as demonstrated by the co-localization of both (F/A)A-ALP and (F/A)RS-ALP with Vph1p on the vacuolar membrane (Fig. 1 B, c, h, e, and j). Like (F/A)A-ALP (Nothwehr et al., 1993), (F/ A)RS-ALP was constructed by mutation of the two phenylalanine residues within the FXFXD motif to alanine residues. The differential localization of RS-ALP and (F/ A)RS-ALP (Fig. 1 B, d and e) demonstrates that it is the FXFXD motif that allows RS-ALP to achieve its TGN localization and in its absence, the protein behaves like ALP and is delivered to the vacuole.
Neither RS-ALP nor (F/A)RS-ALP Depend on VPS45 for Their Localization
At the TGN, recycling Golgi membrane proteins and vacuolar proteins that follow the VPS-dependent (or CPY) pathway to the vacuole enter transport vesicles whose fusion with the PVC is controlled by the Sec1p-like protein Vps45p (Cowles et al., 1994; Piper et al., 1994, 1997; Bryant et al., 1998). In vps45 mutant cells vacuolar proteins such as the 100-kD subunit of the V-ATPase, Vph1p, become trapped in these Golgi-derived transport vesicles, which are unable to fuse with the PVC (Bryant et al., 1998). This mislocalization can be observed using indirect immunofluorescence microscopy where proteins caught in these vesicles display a diffuse staining pattern (Piper et al., 1994, 1997; Bryant et al., 1998). Golgi membrane proteins, such as the CPY receptor Vps10p, that continually cycle between the TGN and the PVC, also enter into Vps45p-controlled vesicles and consequently Vps10p localizes to diffuse vesicular structures in vps45 mutant cells (Bryant et al., 1998). In contrast to this, proteins that follow the alternative, or ALP, pathway from the TGN to the vacuole do not depend on Vps45p to reach their final destination and are localized to the vacuolar membrane in vps45 mutant cells (Piper et al., 1997). This is demonstrated in Fig. 2 A, which shows that ALP was delivered to the vacuolar membrane in vps45Δ cells (Fig. 2 A, a; Piper et al., 1997). In these same cells, in contrast to the vacuolar staining pattern observed in wild-type cells, Vph1p displayed a disperse, clearly non-vacuolar staining pattern consistent with its entrapment inside the vesicles that accumulated in vps45Δ cells (compare Fig. 1 B, f with Fig. 2 A, d). To determine whether the vacuolar proteins (F/A)A-ALP or (F/ A)RS-ALP entered into Vps45p-controlled vesicles, we immunolocalized these proteins in vps45Δ cells. Like Vph1p, (F/A)A-ALP (a version of A-ALP that cannot recycle from the PVC back to the Golgi and consequently travels to the vacuole via the CPY pathway) accumulated in transport vesicles in vps45Δ cells. This is in contrast to its localization in wild-type cells where it was found on the vacuolar membrane (compare Fig. 1 B, c with Fig. 2 A, b). Fig. 2 A also shows that like ALP, whose trafficking to the vacuole is not dependent on VPS45, (F/A)RS-ALP was localized on the vacuolar membrane while Vph1p accumulated in transport vesicles in vps45Δ cells (compare Fig. 2 A, c and f). These data demonstrate that while both (F/ A)A-ALP and (F/A)RS-ALP transit to the vacuole in wild-type cells, they do so by different routes. (F/A)A-ALP enters the Golgi-derived transport vesicles controlled by Vps45p, whereas (F/A)RS-ALP bypasses these trafficking intermediates as it travels to the vacuole.
Figure 2.
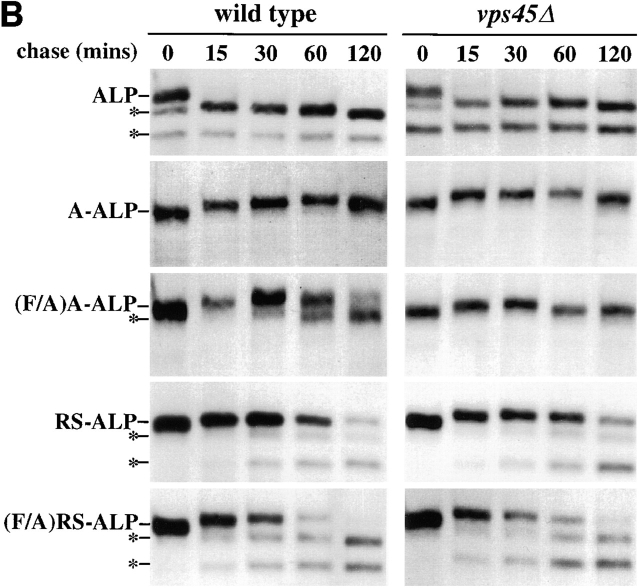
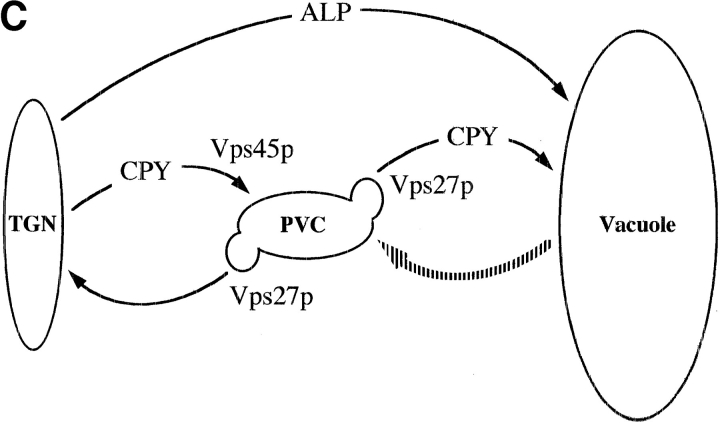
(A) Localization of ALP, (F/A)A-ALP, and (F/A)RS-ALP in vps45Δ cells. NBY83 (vps45Δ-X pho8Δ-X pep4Δ-X) harboring pSN92 (ALP; a, d, and g), pSN100 ((F/A)A-ALP; b, e, and h) or pSN123 ((F/A)RS-ALP; c, f, and i) were prepared for double labeling indirect immunofluorescence using the α-ALP mAb, 1D3-A10 (a–c) and affinity-purified antibodies against the 100-kD subunit of the V-ATPase, Vph1p (d–f), as described in Materials and Methods. Cells were also visualized using DIC microscopy (g–i). (B) Kinetics of processing of ALP, A-ALP, (F/A)A-ALP, RS-ALP, and (F/A)RS-ALP in wild-type and vps45Δ cells. AACY28 (wild type) and NBY68 (vps45Δ-X) pho8Δ-X PEP4 cells harboring pSN92 (ALP), pSN55 (A-ALP), pSN100 ((F/A)A-ALP), pSN97 (RS-ALP), or pSN123 ((F/A)RS-ALP) were labeled with [35S]Met for 10 min and chased by adding unlabeled methionine and cysteine, each to a final concentration of 50 μg/ ml. At the indicated times, proteins were immunoprecipitated from cell extracts using polyclonal antibodies against ALP. The resulting immunoprecipitates were subjected to SDS-PAGE and fluorography. The products of PEP4-dependent proteolysis are indicated using asterisks. (C) Model depicting the pathways taken by CPY and ALP to the vacuole. CPY reaches the vacuole by firstly transiting through a prevacuolar/endosomal compartment (PVC). Entry of proteins into this compartment requires the product of VPS45. Exit of proteins from the PVC, both back to the TGN and on to the vacuole requires the product of VPS27. ALP follows an alternative pathway to the vacuole bypassing the trafficking intermediates defined by mutations in VPS45 and VPS27. The large shaded arrow depicts the retrograde membrane trafficking pathway proposed to be taken by RS-ALP out of the vacuole to reach the PVC.
The observation that (F/A)RS-ALP, like ALP, does not enter Vps45p-controlled transport vesicles demonstrated that sorting information necessary to enter the alternative pathway to the vacuole was fully functional within this protein. We reasoned that the unmutated RS-ALP protein would have both sorting information to enter the alternative pathway to the vacuole, and a functional retrieval signal. In both wild-type and vps45 mutant cells, RS-ALP displayed a punctate distribution (Fig. 1 B, d; data not shown) consistent with its previous colocalization with the TGN protein Kex2p (Nothwehr et al., 1993). However, it was difficult to quantitatively assess the degree to which RS-ALP had a vesicular pattern in vps45 mutant cells compared with its punctate distribution in wild-type cells (Fig. 1 B, d). Thus, to determine quantitatively whether RS-ALP did bypass Vps45p-controlled vesicles, we performed a series of pulse-chase immunoprecipitation experiments.
Upon delivery to the vacuole, proteins containing the lumenal domain of ALP are processed in a PEP4-dependent manner to lower molecular mass forms (Ammerer et al., 1986; Klionsky and Emr, 1989; Nothwehr et al., 1993). For (F/A)A-ALP, this processing occurred with a half-time of ∼60 min in wild-type cells (Fig. 2 B; Nothwehr et al., 1993). In vps45Δ cells, this processing was blocked since (F/A)A-ALP was trapped inside transport vesicles and unable to gain access to a proteolytically active compartment (Fig. 2 B). As expected from the observation that ALP does not require VPS45 to reach the vacuolar membrane, the kinetics of processing of ALP were similar in wild-type and vps45Δ cells (Fig. 2 B; Piper et al., 1997). Consistent with the vacuolar localization of (F/A)RS-ALP in vps45Δ cells (Fig. 2 A, c), this protein was proteolytically processed with similar kinetics in wild-type and vps45Δ cells (Fig. 2 B). RS-ALP was also processed with similar kinetics in wild-type and vps45Δ cells (Fig. 2 B). These data indicate that RS-ALP does not use VPS45-controlled vesicles as part of its normal cellular itinerary, and like (F/A)RS-ALP, follows the VPS45-independent route out of the TGN.
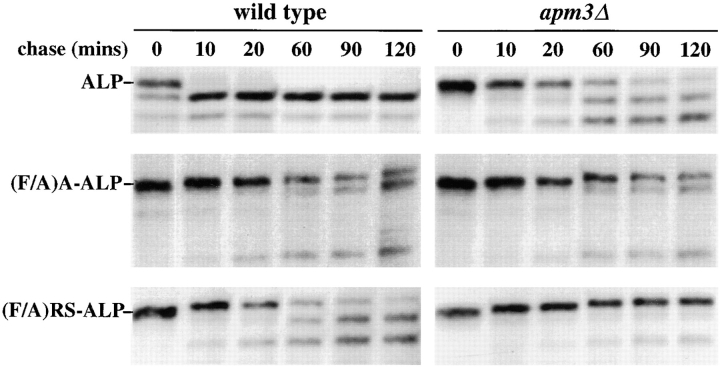
The VPS45-independent route taken by ALP to the vacuole requires the adaptor protein complex AP-3 (Cowles et al., 1997; Stepp et al., 1997). Therefore we tested whether transport of (F/A)RS-ALP and RS-ALP to the vacuole was AP-3 dependent. Pulse-chase immunoprecipitation experiments revealed that the processing of ALP was significantly impaired in cells defective for the AP-3 complex (apm3Δ), as previously reported (Cowles et al., 1997; Stepp et al., 1997). The same set of experiments (Fig. 3) also revealed that the vacuolar delivery of (F/A)A-ALP, which transits to the vacuole through the CPY pathway, is unaffected by the apm3Δ mutation. As observed for ALP, the proteolytic processing of (F/A)RS-ALP was significantly slowed in apm3Δ cells (Fig. 3). The vacuolar delivery of RS-ALP was similarly impaired in apm3Δ cells (data not shown). These data indicate that (F/A)RS-ALP and RS-ALP transport to the vacuole is AP-3 dependent.
Figure 3.
Kinetics of processing of ALP, (F/A)A-ALP, and (F/A) RS-ALP in wild-type and apm3Δ cells. SNY17 (wild-type) and NBY100 (apm3::HIS3) pho8Δ-X PEP4 cells harboring pSN92 (ALP), pSN55 (A-ALP), or pSN123 ((F/A)RS-ALP) were labeled with [35S]Met for 10 min and chased by adding unlabeled methionine and cysteine each to a final concentration of 50 μg/ml. At the indicated times, proteins were immunoprecipitated from cell extracts using polyclonal antibodies against ALP. The resulting immunoprecipitates were subjected to SDS-PAGE and fluorography.
The data presented thus far fit with a model evoking the existence of an as yet undescribed retrograde trafficking pathway out of the vacuole (Fig. 2 C, broken arrow). RS-ALP and (F/A)RS-ALP would travel from the TGN to the vacuole along the alternative pathway taken by ALP, and after recognition of its FXFXD motif, RS-ALP (but not (F/A)RS-ALP) would be retrieved to the TGN via the PVC by the retrograde pathway.
RS-ALP Reaches the Prevacuolar/Endosomal Compartment via the Vacuole
Proteins such as ALP and Vam3p that follow the alternative pathway to the vacuole (Cowles et al., 1997; Darsow et al., 1997; Piper et al., 1997) do not transit through the PVC defined by mutations in the class E VPS gene, VPS27 (Fig. 2 C). vps27 mutants accumulate an exaggerated form of the PVC because of a block of traffic out of this compartment both on to the vacuole and back to the TGN (Piper et al., 1995). This class E compartment contains both endocytosed proteins and recycling late-Golgi membrane proteins as well as vacuolar proteins that follow the VPS-dependent, or CPY, pathway to the vacuole (Piper et al., 1995). Vacuolar proteins such as Vph1p and recycling Golgi membrane proteins such as Vps10p, enter the class E compartment in Golgi-derived, Vps45p-controlled transport vesicles (Bryant et al., 1998) while proteins that follow the alternative pathway to the vacuole bypass this compartment (Piper et al., 1997).
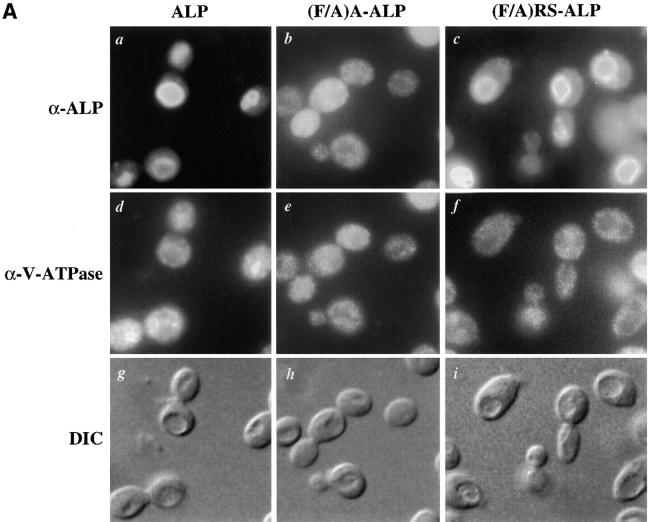
We examined the behavior of RS-ALP and (F/A)RS-ALP in vps27 mutant cells to gain further insight into the trafficking routes of these proteins. Fig. 4 A shows that while Vph1p accumulated in the perivacuolar class E compartment (Fig. 4 A, f–j), ALP was localized to the vacuolar membrane of vps27Δ cells (Fig. 4 A, a; Piper et al., 1997). Entry into the class E compartment from the TGN is controlled by Vps45p (Bryant et al., 1998), and as expected, proteins that get packaged into Vps45p vesicles become trapped in the class E compartment of vps27Δ cells. This is demonstrated in Fig. 4 A where b and c show that A-ALP and (F/A)A-ALP colocalized with Vph1p (g and h) in the class E compartment. By contrast, (F/A)RS-ALP did not require VPS45 to reach the vacuole (Fig. 2 A, c) and further evidence that this protein followed the alternative pathway to the vacuole is shown in Fig. 4 A (e). (F/A)RS-ALP did not accumulate in the class E compartment of vps27Δ cells but instead localized to the vacuole. Interestingly, RS-ALP does localize to the class E compartment of vps27 mutant cells (Fig. 4 A, d; Bryant and Stevens, 1997).
Figure 4.
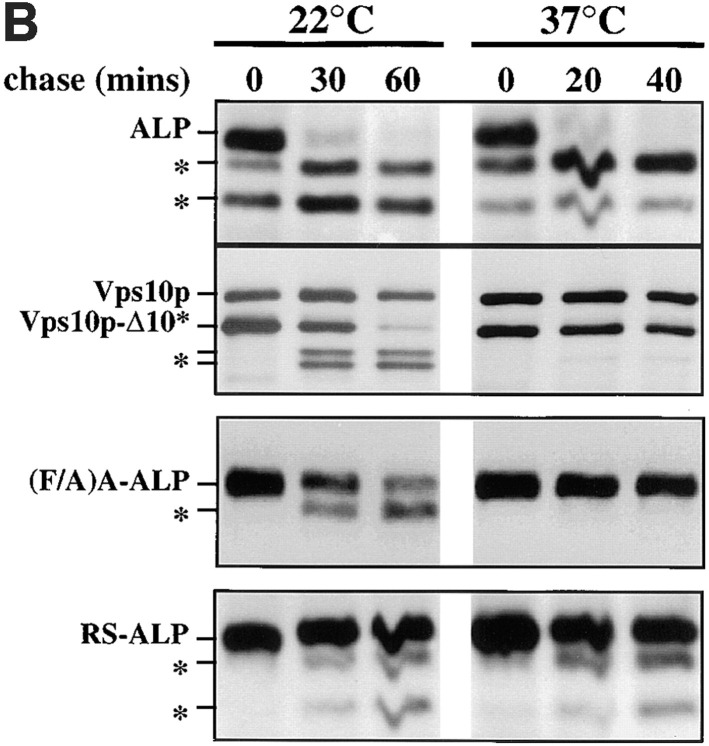
(A) Localization of ALP, A-ALP, (F/A)A-ALP, RS-ALP, and (F/A)RS-ALP in vps27Δ cells. NBY60 (vps27Δ pho8Δ-X pep4-3) cells harboring pSN92 (ALP; a, f, and k), pSN55 (A-ALP; b, g, and l), pSN100 ((F/A)A-ALP; c, h, and m), pSN97 (RS-ALP; d, i, and n), or pSN123 ((F/A)RS-ALP; e, j, and o) were prepared for double-labeling indirect immunofluorescence using the α-ALP mAb, 1D3-A10 (a–e) and affinity-purified antibodies against the 100-kD subunit of the V-ATPase, Vph1p (f–j) as described in Materials and Methods. Cells were also visualized using DIC microscopy (k–o). (B) Rate of PEP4-dependent cleavage of ALP, (F/A)A-ALP, RS-ALP, and Vps10p-Δ10* in vps27-ts cells. RPY103 (VPS10::LEU2::vps10-10* pep4-3 vps27-ts) cells and NBY73 (pho8Δ-X pep4-3 vps27-ts) carrying the GAL1-PEP4 plasmid, pRCP39 and either pNB8 ((F/A)A-ALP) or pNB7 (RS-ALP). Cells were grown in galactose-containing media for 24 h at 22°C, and then shifted to glucose-containing media for 24 h. Before labeling with [35S]Met for 10 min, cells were either maintained at 22°C or shifted to 37°C for 10 min. Chase times used are indicated, after which aliquots were removed from which proteins were immunoprecipitated using α-ALP and α-Vps10p antibodies.
The observation that RS-ALP localizes to the class E compartment is not inconsistent with the observation that it does not travel via the CPY pathway in Vps45p-controlled vesicles. To test the model that RS-ALP visits the vacuole before its arrival at the PVC, we used a regimen in which the PVC of vps27 mutant cells can be made proteolytically inactive while the vacuole remains proteolytically active. This manipulation uses a temperature-sensitive allele of VPS27 and the phenomenon of phenotypic lag (Zubenko et al., 1982). vps27-ts cells harboring a plasmid in which the PEP4 open reading frame was placed under the control of the inducible GAL1 promoter were grown in media containing galactose as their sole carbon source at the permissive temperature of 22°C. We have reported previously that these cells are Pep+ and that when cells are exposed to glucose to shut off production of Pep4p, active vacuolar proteases are flushed from prevacuolar/endosomal compartments (Piper et al., 1997). Shifting these cells to the restrictive temperature of 37°C induces the formation of a proteolytically inactive PVC (Piper et al., 1997). Under this strategy, the vacuole itself remains proteolytically active because of the phenomenon of phenotypic lag, where the autocatalytic activation cycle of protease B results in this and other proteases remaining active for many cell divisions after elimination of Pep4p synthesis (Zubenko et al., 1982; Jones, 1991).
Cells were grown at 22°C in media containing 2% galactose for 24 h, to induce Pep4p production. Expression of PEP4 in these cells was turned off when the cultures were diluted in media containing 2% glucose and incubated at 22°C for an additional 24 h to ensure that Pep4p was flushed out of the biosynthetic pathway to the vacuole. Cells were then either shifted to 37°C for 10 min, or left at 22°C, and subjected to radiolabel pulse/chase analysis to follow the fate of both newly synthesized ALP and newly synthesized Vps10p-Δ10*. Vps10p-Δ10* is a mutant form of the CPY receptor, Vps10p, which lacks the information allowing the protein to cycle between the PVC and the TGN and is instead mislocalized to the vacuole (Cooper and Stevens, 1996). Consequently, Vps10p-Δ10* follows the VPS-dependent pathway to the vacuole and has been shown previously to depend on both VPS27 and VPS45 for its trafficking to the vacuole (Cooper and Stevens, 1996; Piper et al., 1997). Fig. 4 B shows that at 22°C both ALP and Vps10p-Δ10* were proteolytically processed indicating that both had reached the vacuole. These data indicate that the vacuole in these cells is fully capable of processing both ALP and Vps10p-Δ10* despite the fact that there had been no Pep4p biosynthesis for several generations.
At 37°C, the VPS-dependent pathway was blocked as demonstrated by the severe inhibition of Vps10p-Δ10* processing. Since Vps10p-Δ10* localizes to the PVC of vps27 mutant cells (Piper et al., 1997), the observation that no processing occurred indicates that the PVC within these vps27-ts cells at 37°C is proteolytically inactive. In contrast, ALP within the same cells was processed with the same kinetics as observed in wild-type cells, demonstrating that the vacuole was proteolytically active. Fig. 4 B also shows that the processing of (F/A)A-ALP, which is delivered to the vacuole in vps27-ts cells at 22°C (Piper et al., 1997), with 55% of the protein processed after 60 min. The processing of (F/A)A-ALP was blocked at 37°C, with no detectable processing after 40 min, consistent with the protein being trapped within the proteolytically inactive PVC that accumulates within these cells. Recall that like Vps10p-Δ10* and (F/A)A-ALP, RS-ALP becomes trapped in the PVC of vps27Δ cells (Fig. 4 A, d) and vps27-ts cells at 37°C (Bryant and Stevens, 1997). However, unlike Vps10p-Δ10* and (F/A)A-ALP, the processing of RS-ALP was not blocked in vps27-ts cells at 37°C whose PVC had been made proteolytically inactive (40% of RS-ALP has been processed after 40 min at 37°C compared with 35% of the protein after 60 min at 22°C). These data strongly suggest that RS-ALP visits the proteolytically active vacuole before becoming trapped in the PVC of vps27 mutant cells.
RS-ALP and A-ALP Follow Different Intracellular Routes to the PVC
We have previously reported that the entry of proteins such as Vph1p, that follow the CPY pathway to the vacuole, into the class E compartment of vps27 cells requires the function of VPS45 (Bryant et al., 1998). We found that for Vph1p trafficking, VPS45 is epistatic to VPS27 since mutations in VPS45 prevent the accumulation of Vph1p in the PVC of vps27 mutant cells. Similarly, VPS45 is epistatic to VPS27 for Vps10p trafficking, since the recycling receptor becomes trapped inside Golgi-derived transport vesicles and cannot gain entry into the PVC of cells carrying mutations in both VPS45 and VPS27 (Bryant et al., 1998). It is clear that these vps45 vps27 double mutant cells do accumulate a PVC since the endocytosed protein Ste3p, whose trafficking does not depend upon VPS45, accumulates there (Bryant et al., 1998).
While both A-ALP and RS-ALP use continuous retrieval from the PVC to achieve steady-state localization to the TGN (Bryant and Stevens, 1997), it appears that the two proteins are delivered to the PVC via different trafficking pathways. If RS-ALP reaches the PVC via retrograde transport from the vacuole (Fig. 2 C), we predict that while VPS45 function is required for the entry of A-ALP and Vph1p into the PVC (Bryant et al., 1998), RS-ALP will not require Vps45p to enter the same compartment. Fig. 5 shows that both A-ALP and (F/A)A-ALP were prevented from reaching the PVC in vps27Δ vps45Δ double mutants. Neither A-ALP nor (F/A)A-ALP displayed characteristic staining of localization to the class E compartment in these cells, but instead displayed a disperse staining pattern, as seen for Vph1p within the same cells, consistent with their entrapment inside transport vesicles (Fig. 5, b, c, g, and h). These data are consistent with the finding that A-ALP and (F/A)A-ALP use the CPY pathway to transit between the TGN and the PVC (Figs. 2 and 3). Like A-ALP and (F/A)A-ALP, RS-ALP accumulated in the PVC of vps27Δ cells (Fig. 4 A, b–d) but its trafficking to this compartment did not require VPS45, RS-ALP localized to the PVC of vps27Δ vps45Δ double mutants while Vph1p accumulated within VPS45 controlled vesicles within the same cells (compare Fig. 5, d and i).
Figure 5.
Localization of ALP, A-ALP, (F/A)A-ALP, RS-ALP, and (F/A)RS-ALP in vps27Δ vps45Δ double mutant cells. NBY84 (vps27Δ vps45Δ pho8Δ-X pep4-3) cells harboring pSN92 (ALP; a, f, and k), pSN55 (A-ALP; b, g, and l), pSN100 ((F/A)A-ALP; c, h, and m), pSN97 (RS-ALP; d, i, and n), or pSN123 ((F/A)RS-ALP; e, j, and o) were prepared for double labeling indirect immunofluorescence using the α-ALP mAb 1D3-A10 (a–e) and affinity-purified antibodies against the 100-kD subunit of the V-ATPase, Vph1p (f–j), as described in Materials and Methods. Cells were also visualized using DIC microscopy (k–o).
Like ALP, which does not require the function of VPS45 or VPS27 to reach the vacuole (Piper et al., 1997), (F/A)RS-ALP was found on the vacuolar membrane of vps27Δ vps45Δ double mutants (Fig. 5, e). This is consistent with the data presented in Figs. 2 and 3, which show that (F/A)RS-ALP followed the alternative pathway to the vacuole. This, taken with the observation that RS-ALP localized to the PVC of vps27Δ vps45Δ double mutants supports a model in which RS-ALP traffics to the vacuole along the alternative (ALP) pathway before traveling to the PVC and finally back to the TGN as part of its normal cellular itinerary. The unique properties of RS-ALP have thus provided the basis for an assay for the retrograde trafficking step from the vacuole to the prevacuolar/endosomal compartment in yeast.
RS-ALP Cannot Be Retrieved from the Vacuole of vac7 Mutant Cells
The Class III vac mutant vac7 is defective in vacuolar inheritance likely resulting from an inability to perform a scission step necessary to segregate the budding vacuole from the mother into the daughter cell (Bonangelino et al., 1997). We reasoned that the same machinery on the vacuolar membrane might be involved in a trafficking step out of the vacuole, and investigated whether vac7 mutant cells were defective in the transport of RS-ALP out of the vacuole. RS-ALP and A-ALP colocalize with Kex2p to the TGN in wild-type cells (Nothwehr et al., 1993; Bryant and Stevens, 1997) showing the punctate staining pattern in indirect immunofluorescence that is characteristic of localization to this compartment (Fig. 1 B, b and d; Nothwehr et al., 1993). In vac7-1 cells, RS-ALP was found on the vacuolar membrane, colocalizing with the V-ATPase (Fig. 6 B, a and b) while A-ALP still displayed a punctate staining pattern (Fig. 6 C, a and b). Fig. 6 D also shows double labeling of RS-ALP and the TGN protein Vps10p. These two proteins both localize to the TGN in wild-type cells (Nothwehr et al., 1993; Cooper and Stevens, 1996), but in vac7-1 cells, RS-ALP was mislocalized to the vacuolar membrane while Vps10p maintained its punctate, non-vacuolar staining pattern (Fig. 6 D, a and b). Fig. 6 demonstrates that even though vac7-1 cells possessed a morphologically recognizable TGN (as defined by A-ALP and Vps10p), RS-ALP was not found in this organelle as it is in wild-type cells but instead accumulated on the vacuolar membrane. These data suggest that VAC7 is required for a membrane transport pathway out of the vacuole, which RS-ALP uses to achieve its localization to the TGN.
Figure 6.
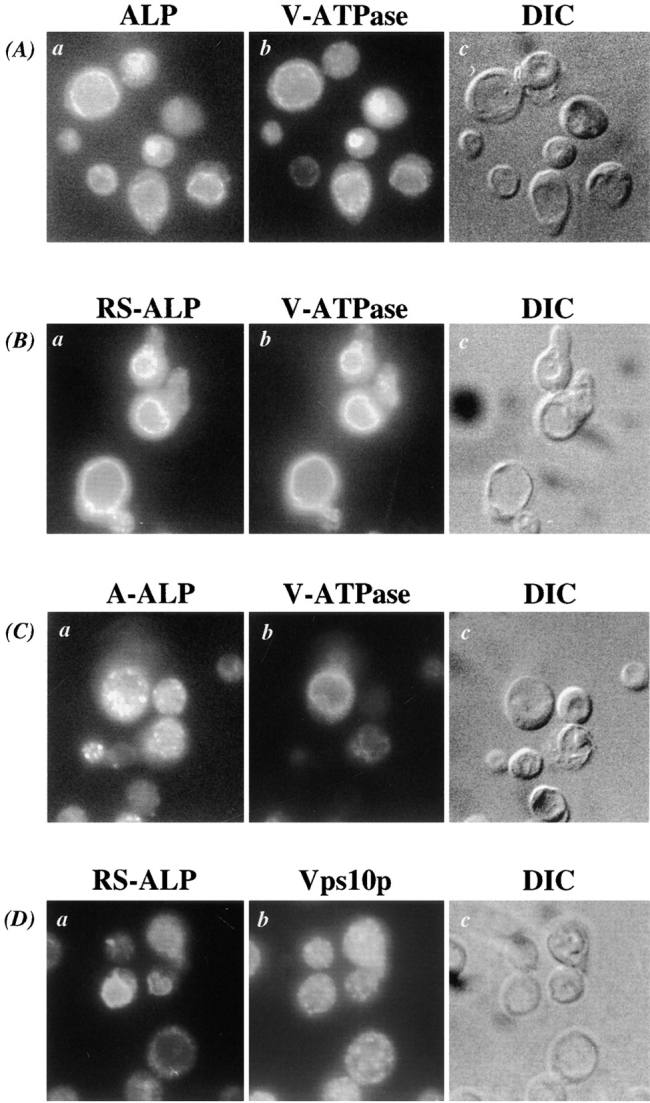
RS-ALP is mislocalized to the vacuolar membrane of vac7 mutant cells. (A) NBY85 (vac7-1 pho8Δ-X pep4Δ-X) cells harboring pSN92 (ALP), (B) pSN97 (RS-ALP), and (C) pSN55 (A-ALP) were prepared for double labeling indirect immunofluorescence using the α-ALP mAb 1D3-A10, and affinity-purified antibodies against the 100-kD subunit of the V-ATPase, Vph1p, as described in Materials and Methods. Cells were also visualized using DIC microscopy (k–o). (D) NBY85 (vac7-1 pho8Δ-X pep4Δ-X) cells harboring pSN97 (RS-ALP) were prepared for double labeling indirect immunofluorescence using the α-ALP mAb 1D3-A10 and affinity-purified antibodies against Vps10p, as described in Materials and Methods. Cells were also visualized using DIC microscopy.
The v-SNARE Vti1p Is Mislocalized in Cells That Block Retrograde Traffic Out of the Vacuole
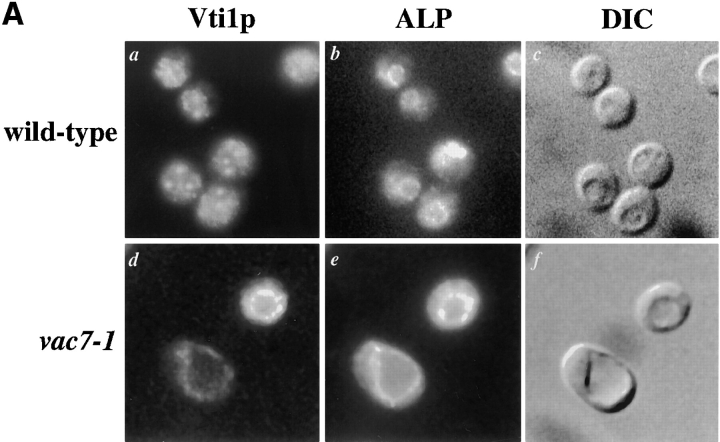
The SNARE hypothesis proposes that interactions between specific v-SNARE molecules on transport vesicles and cognate t-SNAREs on target membranes are involved in controlling the fidelity of membrane fusion (Sollner et al., 1993; Ferro-Novick and Jahn, 1994; Rothman, 1994). In this model, vesicle fusion results in delivery of v-SNAREs to the target membrane and it is likely that these proteins are recycled back to the compartment from where their vesicles bud so that they can be involved in subsequent rounds of targeting. Vti1p is a v-SNARE that has been shown to interact with the t-SNARE Pep12p to control entry of proteins into the PVC (Fischer von Mollard et al., 1997). Vti1p has also been shown to interact with the t-SNARE Sed5p to control protein transport between the ER and the cis-Golgi (Fischer von Mollard et al., 1997; Lupashin et al., 1997). More recently, Vti1p has been shown to interact with Vam3p (Holthius et al., 1998) and to be involved in the alternative pathway taken by ALP and Vam3p to the vacuole (Fischer von Mollard, G., and T.H. Stevens, in preparation). Since Vti1p presumably travels to the vacuole in its role of directing ALP containing trafficking intermediates there, it is likely to be recycled back to the TGN so that it can be involved in further rounds of protein transport. Thus, we identified Vti1p as a candidate for an endogenous cargo protein of the retrograde pathway out of the vacuole. In wild-type cells, Vti1p displayed a punctate immunofluorescence staining pattern (Fig. 7 A, a), but in vac7-1 cells the protein was found on the vacuolar membrane colocalizing with ALP (Fig. 7 A, d and e).
Figure 7.
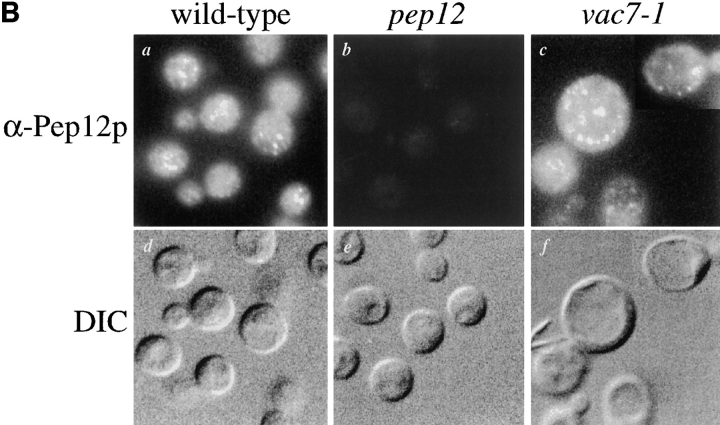
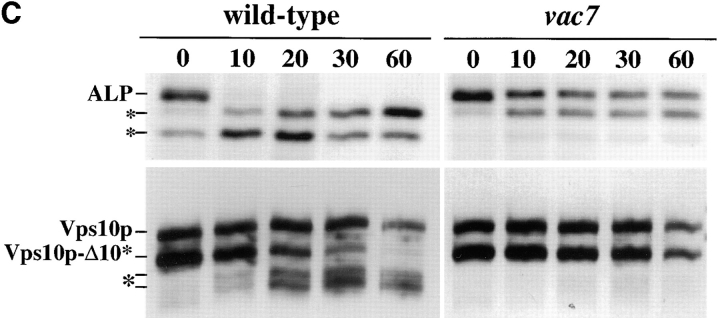
Mislocalization of Vti1p in vac7-1 cells. (A) Wild-type (NBY86; pho8Δ-X pep4Δ-X) and vac7-1 (NBY85; vac7-1 pho8Δ-X pep4Δ-X) cells harboring pSN92 (ALP) were prepared for double labeling indirect immunofluorescence using the α-ALP mAb 1D3-A10 and affinity-purified antibodies against Vti1p, as described in Materials and Methods. (B) Pep12p was immunolocalized in wild-type (NBY86; pho8Δ-X pep4Δ-X) and vac7-1 (NBY85; vac7-1 pho8Δ-X pep4Δ-X) cells harboring pSN92 (ALP) as well as in cells that do not synthesize Pep12p (vpt13), as described in Materials and Methods. In both cases, cells were also visualized using DIC microscopy. (C) Kinetics of processing of ALP and Vps10p-Δ10* in vac7-1 cells. SEY6210 (wild-type) and LWY2809 (vac7-1) PEP4 cells were labeled with [35S]Met for 10 min and chased by adding unlabeled methionine and cysteine each to a final concentration of 50 μg/ml. At the indicated times proteins were immunoprecipitated from cell extracts using polyclonal antibodies against ALP and Vps10p. The resulting immunoprecipitates were subjected to SDS-PAGE and fluorography. The products of PEP4-dependent proteolysis of both ALP and Vps10p-Δ10* are indicated using asterisks.
The endosomal t-SNARE protein Pep12p (Becherer et al., 1996) was also immunolocalized in vac7-1 cells to determine whether this protein would maintain a non-vacuolar localization. Consistent with subcellular fractionation localization of Pep12p (Becherer et al., 1996), Pep12p displayed a punctate, non-vacuolar staining pattern in wild-type cells (Fig. 7 B, a) (similar to that seen for Vti1p in wild-type cells [Fischer von Mollard et al., 1997; Fischer von Mollard, G., unpublished data]). Wild-type cells contained 30–50 of these punctate structures per cell, not seen in cells that do not produce Pep12p (Fig. 7 B, b). Pep12p was observed to maintain a punctate, non-vacuolar distribution in vac7-1 cells (Fig. 7 B, c) and was not mislocalized to the vacuole, arguing that Pep12p does not use the retrograde pathway controlled by VAC7 to maintain its localization.
Vti1p has been shown to be involved in both the VPS-dependent, or CPY pathway, and the alternative, or ALP pathway to the vacuole (Fischer von Mollard et al., 1997; Fischer von Mollard, G., and T.H. Stevens, manuscript in preparation). Since vac7 mutant cells accumulate Vti1p on the vacuolar membrane, vac7 mutant cells could be defective for transport along both the ALP and CPY pathways. In fact, it has been reported that vac7 mutant cells accumulate Golgi-modified forms of CPY intracellularly (Gomes de Mesquita et al., 1996). Fig. 7 C shows pulse-chase immunoprecipitation experiments, which revealed that vac7 mutant cells were also defective in processing of Vps10p-Δ10*, a membrane protein that follows the VPS-dependent pathway to the vacuole (Cooper and Stevens, 1996; Piper et al., 1997; Fig. 4 B). In wild-type cells, Vps10p-Δ10* underwent PEP4-dependent processing with a half-time of ∼15 min (Fig. 7 C; Cooper and Stevens, 1996), but in vac7-1 cells, little processing was observed even after chase times of 60 min. Fig. 7 C also shows that the processing of ALP was significantly slower in vac7 mutant cells than in wild-type cells, with unprocessed forms of ALP still present after 60 min of chase. These data suggest that the vacuolar delivery of ALP is slower in vac7 mutant cells than in wild-type cells, but it is worth noting that processed forms of ALP are obvious after longer chase times, consistent with the immunolocalization of ALP to the vacuole in vac7 mutant cells (Fig. 6 A, a; Fig. 7 A, d; and B, d). This defect in both the CPY and the alternative pathway to the vacuole in vac7-1 cells may reflect mislocalization of proteins such as Vti1p in these cells, since Vti1p uses retrograde transport out of the vacuole as part of its normal cellular itinerary (Fig. 7 A).
Discussion
In this study we report that a hybrid protein (RS-ALP) resident to the TGN of the yeast Saccharomyces cerevisiae achieves its localization by transiting through the vacuole (Fig. 8). Directed by sorting information contained within its cytosolic tail, RS-ALP follows the alternative pathway, as taken by ALP, to the vacuole thus bypassing Golgi- derived transport vesicles that carry proteins from the TGN to the PVC. By virtue of the retrieval motif (FXFXD) that it also carries in its cytosolic tail, RS-ALP then uses a retrograde membrane trafficking step out of the vacuole to reach the PVC from where it is delivered back to the TGN. This work has also revealed that VAC7 function is required for membrane traffic out of the vacuole, and that the v-SNARE Vti1p uses this retrograde trafficking pathway as part of its normal cellular itinerary. This study represents the first report of retrograde membrane traffic out of the vacuole in the yeast S. cerevisiae.
Figure 8.
Model for membrane trafficking pathways between the TGN and the vacuole of S. cerevisiae. There are at least two routes taken by proteins from the TGN to the PVC. Proteins that follow the CPY pathway to the vacuole transit through the PVC in a VPS45, VPS27-dependent manner. From the PVC, these proteins are then delivered to the vacuole. Recycling TGN membrane proteins such as A-ALP are retrieved from the PVC to the TGN. The second route from the TGN to the vacuole does not transit through the PVC and is taken by ALP, RS-ALP, and (F/ A)RS-ALP. After delivery to the vacuolar membrane, RS-ALP follows the newly identified retrograde membrane trafficking step in a VAC7-dependent manner by virtue of its FXFXD retrieval motif.
Identification of a Retrograde Membrane Trafficking Step Out of the Vacuole
The yeast vacuole and its mammalian counterpart the lysosome have typically been thought of as terminal destinations for proteins with regard to membrane trafficking (Kornfeld and Mellman, 1989; Bryant and Stevens, 1998). Recently however, evidence that this might not be the case has been building from work carried out on mammalian cells (see Introduction for details), suggesting that the lysosome acts as a donor membrane in protein trafficking and is therefore a more dynamic organelle than has been widely appreciated. Although the possible existence of a retrograde pathway out of the yeast vacuole has been suggested (Wilcox et al., 1992), there has been no experimental test of this idea. In this study, we have taken advantage of the unique properties of a hybrid protein, RS-ALP (Nothwehr et al., 1993) to demonstrate the existence of a retrograde membrane trafficking step out of the vacuole in the yeast S. cerevisiae. RS-ALP carries a motif (FXFXD) in its cytosolic tail that is sufficient to specify retrieval of the protein from the PVC to the TGN, affording steady state localization to the TGN (Bryant and Stevens, 1997). RS-ALP was constructed in such a way that information sufficient to direct ALP away from the CPY pathway and into the alternative pathway taken by ALP to the vacuole remains present (Nothwehr et al., 1993; Piper et al., 1997).
The transport of ALP, RS-ALP, and (F/A)RS-ALP to the vacuole has been found to be Vps45p independent but requires the adaptor protein complex AP-3. Although ALP, RS-ALP, and (F/A)RS-ALP all travel to the vacuole along the alternative pathway, they are not all proteolytically processed with the same kinetics. Vowels and Payne (1998) recently identified the amino acid residues LV as being the most important signal within the ALP cytosolic domain for targeting into the alternative pathway. The residues LV are missing in (F/A)RS-ALP, and although it is clear that this protein uses the alternative pathway (as does a version of ALP lacking residues 2–21 and therefore the LV signal [Piper et al., 1997]), its slower transport rate to the vacuole likely results from its compromised targeting information. Like (F/A)RS-ALP, RS-ALP also lacks the LV motif, and therefore enters the alternative pathway at a rate slower than ALP, but likely at the same rate as (F/A)RS-ALP. However, RS-ALP is processed in wild-type cells with even slower kinetics than (F/A)RS-ALP. This difference can be explained if the signal-dependent retrieval from the vacuole occurs rapidly enough to prevent RS-ALP from becoming fully processed during one round of transit through the vacuole.
The AP-3–dependent, alternative pathway to the vacuole is unaffected by mutations in either VPS45 or VPS27, which control transit through the PVC (Cowles et al., 1994, 1997; Piper et al., 1994, 1995, 1997; Bryant et al., 1998). We have demonstrated that the trafficking of RS-ALP occurs independently of Vps45p and that it reaches the PVC by first traveling through the proteolytically active vacuole. The observation that RS-ALP localizes to the PVC of vps27 mutant cells (Bryant and Stevens, 1997), whereas a mutant version of the same protein (F/A)RS-ALP is found on the vacuolar membrane, was crucial to the formation of our model in which RS-ALP reaches the PVC from the vacuole via a retrograde membrane trafficking step (Fig. 8).
Although both RS-ALP and the hybrid protein A-ALP achieve TGN localization through continual retrieval from the PVC (Bryant and Stevens, 1997), the route taken by the two proteins to reach the PVC differs. A-ALP consists of the cytosolic domain of the TGN protein DPAP A fused to the transmembrane and lumenal domains of ALP (Nothwehr et al., 1993). A-ALP and its derivative (F/A)A-ALP leave the TGN by entering the CPY pathway out of the Golgi (Fig. 8, pathway 1). Fusion of the vesicles that these proteins enter with the PVC is controlled by the Sec1p-like protein Vps45p. After recognition of its FXFXD motif, A-ALP is recycled from the PVC back to the TGN (Fig. 8, pathway 2) and thus achieves steady state localization to the TGN (Nothwehr et al., 1993; Bryant and Stevens, 1997). Since it does not carry an FXFXD motif, (F/A)A-ALP cannot be retrieved from the PVC to the TGN, but instead travels on from the PVC to the vacuole (Bryant and Stevens, 1997; Fig. 8, pathway 3). In contrast to this, RS-ALP and (F/A)RS-ALP do not enter Vps45p controlled vesicles and instead follow the alternative pathway to the vacuole (Fig. 8, pathway 4). After delivery to the vacuolar membrane, the FXFXD motif of RS-ALP directs its retrograde transport from the vacuole to the PVC (Fig. 8, pathway 5) from where it follows the same pathway as A-ALP to return back to the TGN (Fig. 8, pathway 2). As a result of ablation of the FXFXD motif, (F/A)RS-ALP is not retrieved from the vacuole to the PVC and is therefore localized to the vacuolar membrane of both wild-type and vps27 mutant cells.
This model can be used to explain why RS-ALP undergoes processing by vacuolar proteases in wild-type cells, whereas the CPY receptor, Vps10p, which leaves the TGN and enters the PVC with similar kinetics to RS-ALP, is not exposed to vacuolar proteases (Bryant and Stevens, 1997). RS-ALP becomes processed as it transits through the vacuole en route to the PVC, whereas Vps10p enters the PVC in Vps45p controlled vesicles (Bryant et al., 1998). These data also suggest that the levels of active vacuolar proteases contained within the PVC are not as high as those found in the vacuole.
Machinery and Physiological Relevance of Retrograde Vacuolar Transport
We have demonstrated that mutations in the class III VAC gene VAC7 (Gomes de Mesquita et al., 1996; Bonangelino et al., 1997) cause mislocalization of RS-ALP to the vacuolar membrane. This implies that Vac7p, a vacuolar protein required for vacuolar inheritance and normal vacuolar morphology (Bonangelino et al., 1997), is required for the transport of RS-ALP from the vacuole to the PVC.
The identification of the v-SNARE Vti1p (Fischer von Mollard et al., 1997) as a cargo molecule of the retrograde membrane trafficking pathway out of the vacuole is important since it provides some insight into the physiological relevance of this pathway. Vti1p is required for the CPY pathway, where it interacts with the t-SNARE Pep12p to control the entry of proteins into the PVC (Fischer von Mollard et al., 1997). In addition, Vti1p is required for the trafficking of ALP to the vacuole as demonstrated by the observation that cells carrying a conditional allele of VTI1 accumulate unprocessed ALP under restrictive conditions (Fischer von Mollard, G., and T.H. Stevens, manuscript in preparation). Like RS-ALP, Vti1p accumulates on the vacuolar membrane of vac7 mutant cells. In its role of directing trafficking intermediates to the vacuole along the ALP pathway at least a portion of Vti1p must be transported to the vacuolar membrane. Our observation that Vti1p follows the retrograde trafficking step defined by mutations in vac7 allows us to propose a mechanism for recycling of this v-SNARE to allow it to be involved in further rounds of targeting. Although vac7 mutant cells do not secrete CPY as might be expected from their mislocalization of Vti1p, they do accumulate Golgi-modified, or p2, precursor forms of CPY intracellularly consistent with a defect in TGN to PVC trafficking (Gomes de Mesquita et al., 1996; our unpublished data). In addition, vac7 mutant cells are defective in the processing of ALP as well as in that of Vps10p-Δ10*, a membrane protein marker of the CPY pathway (Cooper and Stevens, 1996; Piper et al., 1997).
While vac7 mutant cells mislocalize Vti1p, overexpression of VTI1 is not sufficient to suppress these processing phenotypes (Bryant, N.J., and T.H. Stevens, unpublished data). This suggests that mislocalization of Vti1p alone is not responsible for the trafficking defects displayed by vac7 mutants. Such an observation is not surprising since there are likely to be additional proteins that travel to the vacuole along with Vti1p by the alternative pathway and it is easy to imagine that these molecules will also be recycled using retrograde transport out of the vacuole. VTI1 is unusual among genes involved in vacuolar protein sorting in that it is an essential gene (Fischer von Mollard et al., 1997). The essential nature of VTI1 arises from the involvement of Vti1p in ER to cis-Golgi trafficking though interactions with the t-SNARE Sed5p (Fischer von Mollard et al., 1997; Lupashin et al., 1997). Although vac7 mutant cells mislocalize Vti1p to the vacuole, these cells are still viable (although it is worth noting that vac7Δ cells do display a slow growth phenotype; Bonangelino et al., 1997). It may be that newly synthesized Vti1p in these cells is sufficient to allow enough membrane traffic to occur through the cis-Golgi to sustain life.
Our studies with RS-ALP and its derivative (F/A)RS-ALP indicate that the delivery of RS-ALP from the vacuole to the PVC depends on the FXFXD motif. There is no such motif within the cytosolic sequence of Vti1p (Fischer von Mollard et al., 1997), which suggests that there are at least two ways of entering this pathway, or perhaps overlapping signals. A similar phenomenon is seen with the signal-dependent alternative pathway taken by ALP to the vacuole (Bryant and Stevens, 1998). The t-SNARE Vam3p also follows this pathway and yet there are no obvious similarities between the sequences of ALP and Vam3p (Wada et al., 1997). To date, very little is known regarding the trafficking of SNARE proteins and it is likely that as more information regarding the intracellular pathways followed by these proteins is uncovered, signals that control the trafficking of Vam3p, Vti1p, and SNARE proteins in general will be identified.
Acknowledgments
We thank S. Nothwehr for the construction of pSN123 and C. Bonangelino for strain LWY2809. We are grateful to L. Graham for her generous supply of affinity-purified antibodies against Vph1p and discussions regarding this work. S. Gerrard is thanked for her help with photography, and we are grateful to members of the Stevens lab for discussion of this work. We would also like to thank G. Fischer von Mollard for sharing her unpublished data and supplying us with affinity-purified antibodies against Vti1p.
This work was supported by National Institutes of Health grants GM 32448 to T.H. Stevens and GM 50403 to L.S. Weisman, and an American Cancer Society grant CSM 87938 to R.C. Piper.
Abbreviations used in this paper
- ALP
alkaline phosphatase
- CPY
carboxypeptidase Y
- DIC
differential interference contrast
- DPAP
dipeptidyl aminopeptidase
- PVC
prevacuolar/endosomal compartment
- SNARE
SNAP receptor
- V-ATPase
vacuolar ATPase
- VAC
vacuolar inheritance
- VPS
vacuolar protein sorting
Footnotes
Address all correspondence to T.H. Stevens, Institute of Molecular Biology, University of Oregon, Eugene, OR 97403-1229. Tel.: (541) 346-5884. Fax: (541) 346-4854. E-mail: stevens@molbio.uoregon.edu
References
- Akasaki K, Fukuzawa M, Kinoshita H, Furuno K, Tsuji H. Cycling of two endogenous lysosomal membrane proteins, lamp-2 and acid phosphatase, between the cell surface and lysosomes in cultured rat hepatocytes. J Biochem (Tokyo) 1993;114:598–604. doi: 10.1093/oxfordjournals.jbchem.a124223. [DOI] [PubMed] [Google Scholar]
- Akasaki K, Michinhara A, Mibuka K, Fujiwara Y, Tsuji H. Biosynthetic transport of a major lysosomal membrane glycoprotein, lamp-1: convergence of biosynthetic and endocytic pathways occurs at three distinctive points. Exp Cell Res. 1995;220:464–473. doi: 10.1006/excr.1995.1338. [DOI] [PubMed] [Google Scholar]
- Ammerer G, Hunter CP, Rothman JH, Saari GC, Valls LA, Stevens TH. PEP4 gene of Saccharomyces cerevisiaeencodes proteinase A, a vacuolar enzyme required for processing of vacuolar precursors. Mol Cell Biol. 1986;6:2490–2499. doi: 10.1128/mcb.6.7.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M, Sato TK, Banta LM, Emr SD. Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO (Eur Mol Biol Organ) J. 1997;16:1820–1831. doi: 10.1093/emboj/16.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becherer KA, Rieder SE, Emr SD, Jones EW. Novel syntaxin homolog, Pep12p, required for the sorting of lumenal hydrolases to the lysosome-like vacuole in yeast. Mol Biol Cell. 1996;7:579–594. doi: 10.1091/mbc.7.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg T, Gjoen T, Bakke O. Physiological functions of endosomal proteolysis. Biochem J. 1995;307:313–326. doi: 10.1042/bj3070313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonangelino CJ, Catlett NL, Weisman LS. Vac7p, a novel vacuolar protein, is required for normal vacuole inheritance and morphology. Mol Cell Biol. 1997;17:6847–6858. doi: 10.1128/mcb.17.12.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachet V, Raposo G, Amigorena S, Mellman I. Ii chain controls the transport of major histocompatibility complex class II molecules to and from lysosomes. J Cell Biol. 1997;137:51–65. doi: 10.1083/jcb.137.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright NA, Reaves BJ, Mullock BM, Luzio JP. Dense core lysosomes can fuse with late endosomes and are re-formed from the resultant hybrid organelles. J Cell Sci. 1997;110:2027–2040. doi: 10.1242/jcs.110.17.2027. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Stevens TH. Two separate signals act independently to localize a yeast late Golgi membrane protein through a combination of retrieval and retention. J Cell Biol. 1997;136:287–297. doi: 10.1083/jcb.136.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant NJ, Stevens TH. Vacuole biogenesis in Saccharomyces cerevisiae: Protein transport pathways to the yeast vacuole. Microbiol Mol Biol Rev. 1998;62:230–247. doi: 10.1128/mmbr.62.1.230-247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant NJ, Piper RC, Gerrard SR, Stevens TH. Traffic into the prevacuolar/endosomal compartment of Saccharomyces cerevisiae: a VPS45-dependent intracellular route and a VPS45-independent, endocytic route. Eur J Cell Biol. 1998;75:43–52. doi: 10.1016/S0171-9335(98)80016-2. [DOI] [PubMed] [Google Scholar]
- Cooper AA, Stevens TH. Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J Cell Biol. 1996;133:529–541. doi: 10.1083/jcb.133.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles CR, Emr SD, Horazdovsky BF. Mutations in the VPS45 gene, a SEC1homologue, result in vacuolar protein sorting defects and accumulation of membrane vesicles. J Cell Sci. 1994;107:3449–3459. doi: 10.1242/jcs.107.12.3449. [DOI] [PubMed] [Google Scholar]
- Cowles CR, Snyder WB, Burd CG, Emr SD. Novel Golgi to vacuole delivery pathway in yeast: identification of a sorting determinant and required transport component. EMBO (Eur Mol Biol Organ) J. 1997;16:2769–2782. doi: 10.1093/emboj/16.10.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow T, Rieder SE, Emr SD. A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J Cell Biol. 1997;138:517–529. doi: 10.1083/jcb.138.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeDuve, C. 1963. The lysosome concept. In Lysosomes: Ciba Foundation Symposium. A.V.S. de Reuck, and M.P. Cameron, editors. Churchill, London. 1–35.
- Ferro-Novick S, Jahn R. Vesicle fusion from yeast to man. Nature. 1994;370:191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G, Nothwehr SF, Stevens TH. The yeast v-SNARE Vti1p mediates two vesicle transport pathways through interactions with the t-SNAREs Sed5p and Pep12p. J Cell Biol. 1997;137:1511–1524. doi: 10.1083/jcb.137.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzusoff A, Redding K, Crosby J, Fuller RS, Schekman R. Localization of components involved in protein transport and processing through the yeast Golgi apparatus. J Cell Biol. 1991;112:27–37. doi: 10.1083/jcb.112.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF–EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes de Mesquita DS, van den Hazel HB, Bouwman J, Woldringh CL. Characterization of new vacuolar segregation mutants, isolated by screening for loss of proteinase B self-activation. Eur J Cell Biol. 1996;71:237–247. [PubMed] [Google Scholar]
- Griffiths G, Holflack B, Simons K, Mellman I, Kornfeld S. The mannose-6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- Holthuis JCM, Nichols BJ, Dhruvakumar S, Pelham HRB. Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO (Eur Mol Biol Organ) J. 1998;17:113–126. doi: 10.1093/emboj/17.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honing S, Sondoval IV, Figura K. A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tryosinase mediates selective binding of AP-3. EMBO (Eur Mol Biol Organ) J. 1998;17:1304–1314. doi: 10.1093/emboj/17.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horazdovsky BF, Busch GR, Emr SD. VPS21encodes a rab5-like GTP binding protein that is required for the sorting of yeast vacuolar proteins. EMBO (Eur Mol Biol Organ) J. 1994;13:1297–1309. doi: 10.1002/j.1460-2075.1994.tb06382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Geuze HJ. Intracellular trafficking of lysosomal membrane proteins. Bioessays. 1996;18:379–389. doi: 10.1002/bies.950180508. [DOI] [PubMed] [Google Scholar]
- Jahrous A, Storrie B, Griffiths G, Desjardins M. Evidence for retrograde traffic between terminal lysosomes and the prelysosomal/late endosome compartment. J Cell Sci. 1994;107:145–157. doi: 10.1242/jcs.107.1.145. [DOI] [PubMed] [Google Scholar]
- Jones EW. Three proteolytic systems in the yeast Saccharomyces cerevisiae. . J Biol Chem. 1991;266:7963–7966. [PubMed] [Google Scholar]
- Klionsky DJ, Emr SD. Membrane protein sorting: biosynthesis, transport and processing of yeast vacuolar alkaline phosphatase. EMBO (Eur Mol Biol Organ) J. 1989;8:2241–2250. doi: 10.1002/j.1460-2075.1989.tb08348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Kunkel TA, Roberts JD, Zakour RA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Liou W, Geuze HJ, Geelen MJ, Slot JW. The autophagic and endocytic pathways converge at the nascent autophagic vacuoles. J Cell Biol. 1997;136:61–70. doi: 10.1083/jcb.136.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Fambrough DM. Cycling of the integral membrane proteins, LEP100, between plasma membrane and lysosomes: kinetic and morphological analysis. Cell. 1987;49:669–677. doi: 10.1016/0092-8674(87)90543-5. [DOI] [PubMed] [Google Scholar]
- Ludwig T, Griffiths G, Hoflack B. Distribution of newly synthesized lysosomal enzymes in the endocytic pathway of normal rat kidney cells. J Cell Biol. 1991;115:1561–1572. doi: 10.1083/jcb.115.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupashin VV, Pokrovskaya ID, McNew JA, Waters MG. Characterization of a novel yeast SNARE protein implicated in Golgi retrograde traffic. Mol Biol Cell. 1997;8:2659–2676. doi: 10.1091/mbc.8.12.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullock BM, Bright NA, Fearon CW, Gray SR, Luzio JP. Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent. J Cell Biol. 1998;140:591–601. doi: 10.1083/jcb.140.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr SF, Stevens TH. Sorting of membrane proteins in the yeast secretory pathway. J Biol Chem. 1994;269:10185–10188. [PubMed] [Google Scholar]
- Nothwehr SF, Roberts CJ, Stevens TH. Membrane protein retention in the yeast Golgi apparatus: dipeptidyl aminopeptidase A is retained by a cytoplasmic signal containing aromatic residues. J Cell Biol. 1993;121:1197–1209. doi: 10.1083/jcb.121.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr SF, Conibear E, Stevens TH. Golgi and vacuolar membrane proteins are transported to the plasma membrane and then to the vacuole in vps1mutant yeast cells. J Cell Biol. 1995;129:35–46. doi: 10.1083/jcb.129.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Whitters EA, Stevens TH. Yeast Vps45p is a Sec1p-like protein required for the consumption of vacuole-targeted, post-Golgi transport vesicles. Eur J Cell Biol. 1994;65:305–318. [PubMed] [Google Scholar]
- Piper RC, Cooper AA, Yang H, Stevens TH. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. . J Cell Biol. 1995;131:603–617. doi: 10.1083/jcb.131.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Bryant NJ, Stevens TH. The membrane protein alkaline phosphatase is delivered to the vacuole by a route that is distinct from the VPS-dependent pathway. J Cell Biol. 1997;138:531–545. doi: 10.1083/jcb.138.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C, Strous GJ, Crapo JD, Geuze HJ, Slot JW. The differential degradation of two cytosolic proteins as a tool to monitor autophagy in hepatocytes by immunocytochemistry. J Cell Biol. 1993;120:897–908. doi: 10.1083/jcb.120.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves BJ, Bright NA, Mullock BM, Luzio JP. The effect of wortmannin on the localisation of lysosomal type I integral membrane glycoproteins suggests a role for phosphoinositide 3-kinase activity in regulating membrane traffic late in the endocytic pathway. J Cell Sci. 1996;109:749–762. doi: 10.1242/jcs.109.4.749. [DOI] [PubMed] [Google Scholar]
- Redding K, Holcomb C, Fuller RS. Immunolocalization of Kex2 protease identifies a putative late Golgi compartment in the yeast Saccharomyces cerevisiae. . J Cell Biol. 1991;113:527–538. doi: 10.1083/jcb.113.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JS, Klionsky DJ, Banta LM, Emr SD. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol. 1988;8:4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Rothman JH, Howald I, Stevens TH. Characterization of genes required for protein sorting and vacuolar function in the yeast Saccharomyces cerevisiae. . EMBO (Eur Mol Biol Organ) J. 1989;8:2057–2065. doi: 10.1002/j.1460-2075.1989.tb03614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J.E., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. 2nd Edition. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 53–105.
- Sherman, F., G.R. Fink, and J. Hicks. 1986. Methods in Yeast Genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York. 146–153.
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. . Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Kruger B, Stenmark H, Dusterhoft A, Philippsen P, Yoo JS, Gallwitz D, Zerial M. Role of three rab5-like GTPases, Ypt51p, Ypt52p, and Ypt53p, in the endocytic and vacuolar protein sorting pathways of yeast. J Cell Biol. 1994;125:283–298. doi: 10.1083/jcb.125.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Stack JH, Horazdovsky B, Emr SD. Receptor-mediated protein sorting to the vacuole in yeast: roles for a protein kinase, a lipid kinase and GTP-binding proteins. Annu Rev Cell Dev Biol. 1995;11:1–33. doi: 10.1146/annurev.cb.11.110195.000245. [DOI] [PubMed] [Google Scholar]
- Stepp JD, Huang K, Lemmon SK. The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J Cell Biol. 1997;139:1761–1774. doi: 10.1083/jcb.139.7.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B, Desjardins M. The biogenesis of lysosomes: is it a kiss and run continuous fusion and fission process? . Bioessays. 1996;18:895–903. doi: 10.1002/bies.950181108. [DOI] [PubMed] [Google Scholar]
- Traub LM, Bannykh SI, Rodel JE, Aridor M, Balch WE, Kornfeld S. AP-2–containing clathrin coats assemble on mature lysosomes. J Cell Biol. 1996;135:1801–1814. doi: 10.1083/jcb.135.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deurs B, Holm PK, Kayser L, Sandvig K. Delivery to lysosomes in the human carcinoma cell line HEp-2 involves an actin filament- facilitated fusion between mature endosomes and preexisting lysosomes. Eur J Cell Biol. 1995;66:309–323. [PubMed] [Google Scholar]
- Vowels JJ, Payne GS. A dileucine-like sorting signal directs transport into an AP-3-dependent, clathrin-independent pathway to the yeast vacuole. EMBO (Eur Mol Biol Organ) J. 1998;17:2482–2493. doi: 10.1093/emboj/17.9.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y, Nakamura N, Ohsumi Y, Hirata A. Vam3p, a new member of syntaxin related protein, is required for vacuolar assembly in the yeast Saccharomyces cerevisiae. . J Cell Sci. 1997;110:1299–1306. doi: 10.1242/jcs.110.11.1299. [DOI] [PubMed] [Google Scholar]
- Wilcox CA, Redding K, Wright R, Fuller RS. Mutation of a tyrosine localization signal in the cytosolic tail of yeast Kex2 protease disrupts Golgi retention and results in default transport to the vacuole. Mol Biol Cell. 1992;3:1353–1371. doi: 10.1091/mbc.3.12.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DH, Fink GR. Proteinase C (carboxypeptidase Y) mutant of yeast. J Bacteriol. 1975;123:1150–1156. doi: 10.1128/jb.123.3.1150-1156.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubenko GS, Park FJ, Jones EW. Genetic properties of mutations at the PEP4 locus in Saccharomyces cerevisiae. . Genetics. 1982;102:679–690. doi: 10.1093/genetics/102.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]