Abstract
The relative importance of muscle activity versus neurotrophic factors in the maintenance of muscle differentiation has been greatly debated. Muscle biopsies from spinal cord injury patients, who were trained with an innovative protocol of functional electrical stimulation (FES) for prolonged periods (2.4–9.3 years), offered the unique opportunity of studying the structural recovery of denervated fibers from severe atrophy under the sole influence of muscle activity. FES stimulation induced surprising recovery of muscle structure, mass, and force even in patients whose muscles had been denervated for prolonged periods before the beginning of FES training (up to 2 years) and had almost completely lost muscle-specific internal organization. Ninety percent (or more) of the fibers analyzed by electron microscopy showed a striking recovery of the ultrastructural organization of myofibrils and Ca2+-handling membrane systems. This functional/structural restoration follows a pattern that mimics some aspects of normal muscle differentiation. Most importantly, the recovery occurs in the complete absence of motor and sensory innervation and of nerve-derived trophic factors, that is, solely under the influence of muscle activity induced by electrical stimulation.
Keywords: atrophy, denervation, spinal cord injury
Bidirectional communication between muscle fibers and motor neurons is extremely important for the development and maintenance of the entire neuromuscular apparatus (1). The interdependence of the two systems becomes obvious when this communication is interrupted, that is, in neuropathological conditions or as a result of traumatic events such as spinal cord injuries (SCIs) or damage of peripheral nerves (2, 3).
In skeletal muscle, lack of innervation causes severe alterations of fiber properties: general disarrangement of internal structure accompanied by functional impairment and followed by complete degeneration (4–6). Motor neurons regulate the properties of muscles directly through trophic factors and indirectly through activity. However, the relative importance of neurotrophic factors vs. muscle activity in the maintenance of muscle mass, contractile properties, and fiber-type characteristics have been greatly debated. It has been proposed that, during early development, fiber diversity is independent of innervation (7, 8). Conversely, in the adult, specific properties, such as differential expression of protein isoforms in different fiber types, internal organization of membrane systems, and metabolic machinery, are influenced by innervation. Cross-innervation experiments of fast and slow muscles with motor neurons having different firing patterns (tonic vs. fast) resulted in a reciprocal transformation of some muscle fiber properties (9, 10). In all these early experiments, it was not possible to separate the relative importance of neurotrophic factors vs. contractile activity. The effects of denervation cannot be reproduced just by a reduced level of activity, that is, disuse or decreased use in the presence of innervation, which suggests that neurotrophic factors are of importance in controlling fiber properties (11). However, in the absence of other markers of fiber-type specificity, this aspect needs further clarification.
There are several findings, however, supporting the idea that activity represents an important cofactor in controlling muscle characteristics. In fact, the effects of cross-innervation of fast muscles with slow motor neurons can be approximated in normally innervated muscle by a superimposed pattern of chronic electrical stimulation with a tonic pattern of activity (12–15). In addition, in dysgenic and dyspedic mice, two animal models lacking the key protein of the excitation-contraction (EC) coupling mechanism, which are normally innervated and capable of action potentials, muscle fiber differentiation is severely impaired, presumably because of the complete lack of contractile activity (16, 17). Further evidence suggesting that activity is indeed important was provided in tenotomized muscle. Reduced muscle activity in rat soleus resulted in a slow-to-fast shift of contractile properties (18) and the slow kinetics of contraction on such tenotomized muscle could be maintained by applying a slow pattern of stimulation (19). In the same vein, a slow-to-fast transformation can be induced by chronic high-frequency stimulation in denervated muscle (20). Finally, electrical stimulation is somehow effective in limiting denervation-induced atrophy and in improving muscle force and recovery after reinnervation (21–23).
Studies aiming to rescue function in denervated muscle by using electrically evoked activity were encouraging (21–23). However, these experiments were performed mostly in animal models and after relatively short periods of denervation, when atrophy and degeneration is minor. This leaves an open question: can extreme muscle wasting due to prolonged periods of denervation be reversed in the absence of innervation? A unique opportunity to explore this question was offered by the availability of human muscle biopsies from paraplegic patients affected by complete lesion of the conus cauda and subjected to direct (functional) electrical stimulation (FES) for prolonged periods (2.4–9.3 years) (24–27). Starting from a severe level of atrophy and in complete absence of both motor and sensory innervation, the electrically stimulated muscle fibers show a surprising structural recovery that resembles some aspects of normal embryonal and postnatal muscle differentiation.
Results
Prolonged Denervation Causes Severe Atrophy.
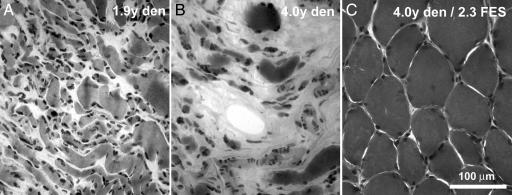
The histological analysis of muscle biopsies of untreated patients show features of classical denervation atrophy (4–6), the severity of which depends on the time elapsed from the injury (Fig. 1 A and B; see also ref. 25 for more detail). Fiber diameters decrease progressively and, at 2 years after injury, most, but not all, fibers have a small diameter [range, 9–27 μm; Table 1 and supporting information (SI) Fig. 5]. The percentage of cross-sectional area in the biopsy occupied by muscle fibers is ≈30% on the average (range, 13–50%). The remaining percentage of cross-sectional area (range, 50–87%) is filled with connective and adipose tissue, which increases with lengthening periods elapsed from injury (SI Fig. 5). However, because exact information on total muscle mass was not available, it is not possible to tell whether connective and adipose tissues actually increase in absolute terms.
Fig. 1.
Denervation-induced atrophy of muscle fibers is reversed by FES. (A and B) Denervation (den) causes progressive atrophy of muscle fibers and a relative increase of connective and adipose tissues. (C) FES treatment greatly increases average diameter of muscle fibers and significantly reduces the relative content of collagen and adipocyte accumulation.
Table 1.
SCI patients
| Patients | Time intervals between SCI and muscle biopsy, yr | Mean fiber diameter ± SD, mm (n) | Fibers analyzed, n | Severely atrophic fibers, n (%) | Atrophic fibers with some cross-striated areas, n (%) |
|---|---|---|---|---|---|
| SCI 1 | 0.9 | 27 ± 8 (210) | 38 | 30 (79) | 8 (21) |
| SCI 2 | 1.3 | 16 ± 10 (208) | 41 | 41 (100) | 0 (0) |
| SCI 3 | 1.8 | 24 ± 18 (250) | 47 | 45 (96) | 2 (4) |
| SCI 4 | 1.9 | 11 ± 8 (218) | 38 | 33 (87) | 5 (13) |
| SCI 5 | 4.0 | 9 ± 11 (217) | 49 | 49 (100) | 0 (0) |
All fibers in SCI patients are severely compromised and atrophic. The mean fiber diameter decreases significantly during the first year of denervation and progresses with increasing times. All the fibers analyzed show evident signs of internal disorganization even if a small percentage of the fibers, which decreases with longer denervation times, do have small islands of aligned myofibrils forming a cross-striation.
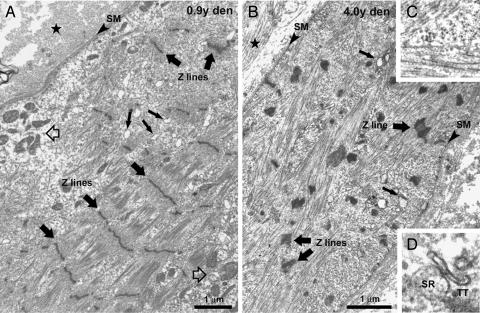
In electron micrographs, severely atrophic muscle fibers are identifiable based on recognizable remnants of contractile material (Fig. 2). The remaining myofibrils are reduced in size and usually discontinuous and/or completely missing from extended areas. Sarcomeres are often altered, with missing M lines and widened and/or streaming Z lines, a feature common to a wide variety of muscle diseases (5). The widened intermyofibrillar spaces contain an amorphous cytoskeletal network with scarce, often clustered, mitochondria (Fig. 2A). The sarcoplasmic reticulum (SR) is incomplete and vacuolated, and transverse (T) tubules are hardly recognizable, except where they associate with elements of the SR to form poorly shaped dyads and triads (Fig. 2D). In fibers where more structural elements are present, the disorganization is always more severe in the subsarcolemmal regions (Fig. 2A).
Fig. 2.
Effects of long-term denervation on skeletal fibers ultrastructure. (A and B) Disarrangement of the internal structure of fibers starts from the periphery and results in complete disruption of the internal organization. (C) Shown is an area with misoriented contractile filaments. (D) Shown is an abnormal SR/T tubule junction. Filled stars, extracellular space; open arrows, mitochondria grouping; small filled arrows, fragmented SR; large filled arrows, Z lines; SM, surface membrane.
The longer the period after injury, the greater the degree of ultrastructural disorganization of the fiber interior. Myofibrils and/or sarcomeres, mitochondria, and T tubule/SR junctions, even though disrupted, are still relatively frequent in patients that have been denervated for shorter periods, but they are quite rare after prolonged denervation. The frequency of the fibers containing small areas of aligned cross-striation decreases progressively with increasing denervation periods (Table 1).
FES Induces a Striking Recovery of the Fiber Architecture.
Histological analysis of biopsies from FES-treated patients (Fig. 1C) show a significant increase in the average fiber diameter (range, 27–48 μm), compared with untreated patients (range, 9–27 μm) (compare Tables 1 and 2). The size distribution of fiber diameters indicates that most fibers present a larger diameter than denervated samples (see SI Fig. 5). The increase in fiber size is accompanied by a significant reduction in the relative content of connective and adipose tissues (Fig. 1C): on the average, only ≈5% (range, 1–26%) of the cross-sectional area (see SI Fig. 5).
Table 2.
FES-trained patients
| Patients | Time intervals between, yr |
Mean fiber diameter ± SD, mm (n) | Fibers analyzed, n | Severely atrophic fibers, n (%) | Partially recovered fibers, n (%) | Recovered fibers, n (%) | ||
|---|---|---|---|---|---|---|---|---|
| SCI and FES | FES and biopsy | SCI and muscle biopsy | ||||||
| FES 1 | 1.2 | 2.4 | 3.6 | 27 ± 25 (152) | 27 | 4 (15) | 9 (39) | 14 (46) |
| FES 2 | 1.7 | 2.3 | 4.0 | 43 ± 19 (216) | 22 | 0 (0) | 8 (36) | 14 (64) |
| FES 3 | 2.0 | 4.3 | 6.3 | 46 ± 18 (140) | 25 | 1 (4) | 3 (12) | 21 (84) |
| FES 4 | 1.9 | 7.7 | 9.6 | 39 ± 23 (176) | 33 | 3 (9) | 5 (17) | 25 (74) |
| FES 5 | 1.3 | 9.3 | 10.6 | 48 ± 12 (107) | 24 | 1 (4) | 2 (9) | 21 (87) |
Most fibers in FES-trained patients are recovered/recovering. FES treatment leads to a significant increase in the average diameter of fibers and in a drastic reduction of severely atrophic ones. Most fibers present a restored contractile apparatus in the large majority of their interior, whereas there is still a percentage of recovering fibers (39% to 9%), presenting areas in which the myofibrils are not completely reorganized.
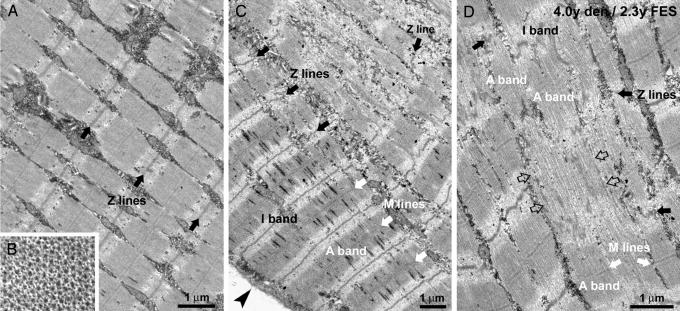
Striking is the restoration of the fiber's internal architecture at the electron microscopy analysis. In FES-treated muscles, the cross-striation covers the large majority of fiber areas visible in thin sections. Myofibrils are quite well aligned with one another (Fig. 3A), sarcomeres have visible Z and M lines and exhibit an ordered double-hexagonal array of thin and thick filaments in cross-section (Fig. 3B). Myofibril restoration, though, is not always complete throughout the cross-section. In some fibers, peripherally located, reorganized regions coexist with central areas in which myofibrils are not completely reassembled (Fig. 3C). Because of the limited availability of tissue we could not determine directly the fiber-type compositions of the regenerating muscles. However, the Z line width is a structural feature that may discriminates between fiber types (28, 29). Considering this parameter, the fibers fall into two separate categories: one with the thinner Z lines (65–75 nm) and the other with wider Z lines (84–97 nm) in both normal and FES-rescued muscles (SI Table 3). Representative images of fibers with thinner and wider Z lines are shown in SI Fig. 6. Interestingly, the number of fibers with a wider Z line, which presumably are more resistant to fatigue, is higher in muscles that have been stimulated for longer periods (FES 4 and 5).
Fig. 3.
FES-induced ultrastructural restoration of myofibrils. (A and B) Rescued fibers present a transversal dark–pale striation (A) and a regular hexagonal pattern of thick and thin filaments (B). (C) In partially recovered fibers, myofibrils are better organized at the fiber periphery (arrowhead). (D) The formation of new myofibrils resembles myofibrillogenesis in normal embryonal differentiation: alignment of filaments (open arrows), preassembly of A bands, appearance of M lines, and formation of Z lines (see Results for more details). Black arrows, Z lines; white arrows, M lines; arrowhead, surface membrane; open arrows, aligned myofilaments, which are not yet assembled into sarcomeres.
Semiquantitative Evaluation of Structural Recovery.
An estimate of the extent of structural recovery induced by FES was obtained by classifying the muscle fibers according to structural parameters indicative of either a deep level of atrophy or of various degrees of recovery. We define as severely atrophic those fibers that have a highly disorganized contractile apparatus, with null, or almost completely missing, striation (Fig. 2). Partially recovered are fibers that present extensive areas of well differentiated myofibrils, but show one or more remaining disorganized regions along the observed length (Fig. 3C). Finally, fully recovered fibers are those fibers presenting well aligned cross-striation throughout the entire sectioned segment (Fig. 3A). Severely atrophic fibers are the large majority in the untreated patients, but they constitute a minor percentage in the FES patients (Tables 1 and 2). Partially recovered fibers constitute a minor percentage of the fibers in FES patients (Table 2), suggesting that most of the fibers are a long way toward full structural recovery by the time the biopsies are taken. Interestingly, the percentage of partially recovered fibers, although small, decreases with increasing time of FES treatment (Table 2), indicating either that more fibers are fully recovered with the longer treatment, or that the fully recovered peripheral ring occupies a larger percentage of the total fiber volume. Finally, fully recovered fibers are the large majority in FES-trained patients.
Possible Sequence of Changes During Intermediate Stages of FES-Induced Recovery.
Partially recovered fibers, that is, containing areas where myofibrils are not completely restored, offered the opportunity of identifying some of the steps leading to sarcomere assembly and restoration of the contractile apparatus (Fig. 3 C and D). Well restored regions are mostly located at the fiber's periphery, whereas disordered domains are central (Fig. 3C) and their frequency decreases with increasing periods of FES (Table 2). This suggests that myofibrillogenesis starts at the fiber's periphery and proceeds centrally. Incomplete sarcomeres have thick filaments that are parallel, but misaligned so that the edges of the A band are not well defined (Fig. 3D). At this stage, the M lines are missing, and discontinuous Z bodies are located in the Z line region of the sarcomere. An increase in the order of thin and thick filaments, appearance of an M line, and of continuous, transversely aligned Z lines complete the assembly of the mature sarcomere (Fig. 3D).
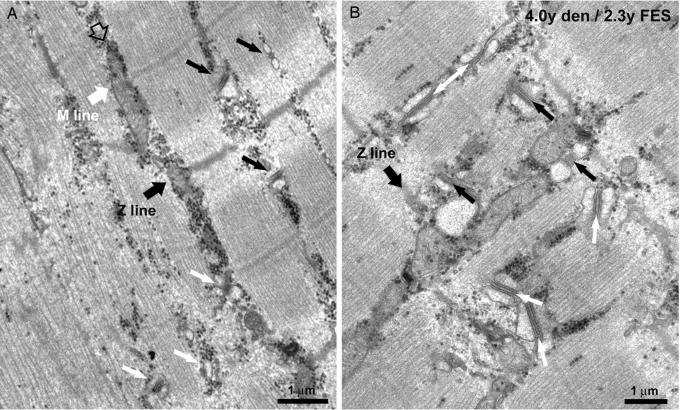
Also the membrane systems involved in the EC coupling mechanism are progressively restored by the FES stimulation of denervated muscles (Fig. 4). The differentiation of a mature T tubule network (see SI Fig. 7 for more detail) is accompanied by the maturation of the SR and by the formation of well differentiated triads, that is, junctions between the SR and the T tubules. The reorganization of the sarcotubular system closely follows the reorganization of the myofibrils: SR/T tubule junctions are more numerous in regions that present well differentiated myofibrils than in areas where myofibrils are incomplete (Fig. 4A). The transversal positioning of triads at the edge of the A bands is likely preceded by a longitudinal/oblique orientation, as it occurs during normal differentiation (Fig. 4B).
Fig. 4.
FES induced restoration of the EC coupling apparatus. (A) Triads are more frequent and better oriented in regions presenting well differentiated myofibrils (black arrows). (B) The transversal positioning of triads at the edge of the A bands (black arrows) is likely preceded by a longitudinal/oblique orientation (white arrows).
Discussion
This study provides a unique opportunity to gain new understanding of the relationship between activity and differentiation/maintenance of muscle fiber in the complete absence of nerve input. Although a significant influence of mechanical activity on muscle properties has long been established (12–15, 18, 19), here, we provide the information that the direct electrical stimulation of human denervated muscle can reverse muscle atrophy, even after extended denervation and inactivity. Starting from a severely degenerated state, the muscles from FES-treated patients studied in the present work achieved an almost complete structural differentiation. These results are of interest both from a basic biological perspective, because this recovery occurs in the total absence of any neural trophic influence, and from a clinical point of view, because the difficulty with poor recovery of long-term denervated muscle has been a long-standing problem (30, 31).
The mechanisms by which the ultrastructure of myofibrils is rescued and the EC coupling machinery is reassembled, in many aspects, recapitulates the normal development. The spread of myofibrillogenesis from periphery to center, the intermediate steps in sarcomere assembly, and the coordinated interplay of the membrane systems with the myofibrils are all steps that have been described as intermediate stages of fiber differentiation in vivo and in vitro (32, 33). Quantitative analysis also detected trends in the data, which are possibly significant. First, the level of recovery improves with increasing times of FES training in terms of how many fibers are rescued and of how complete the recovery is. Second, the presence of fibers with different Z line widths (see SI Table 3 and SI Fig. 6) would indicate that at least one of the fiber-type-specific parameters is exhibited by these muscles. The appearance of a heterogeneous population of fibers in the absence of neurotrophic influence supports the existence of intrinsic myogenic factors in fiber differentiation (7, 8).
One important issue should be discussed, though, to validate our findings: are the small biopsies collected from such a large muscle representative? Biopsies were specifically collected from the central/front area of the thigh area because it was expected to receive the most effective stimulation from the superficially placed electrodes (24, 26). It is possible that, within other areas of the thigh, fibers were not as well rescued as they are in the collected specimens. However, despite the fact that the biopsies are intrinsically somewhat heterogeneous from one patient to another, and are likely not to be representative of the entire thigh muscle, the main findings of this study are still significant. In fact, the recovery of fiber structure is striking (this study) and muscle fibers can produce tension and force when stimulated with FES devices (27).
An important question that still needs to be addressed is whether the structural rescue observed here is caused by de novo formation of fibers or by reactivation of the myogenic program in the preexisting atrophic fibers. The parallel increase in the number of recovering and decrease in number of atrophic fibers in FES-trained patients, as well as the presence of fibers that show a regenerating periphery in concert with an atrophic core, would argue for a restoration of muscle structure and function in preexisting atrophic fibers, even if the possibility of de novo formation cannot be excluded. The role of satellite cells, if any, in rescuing of the atrophic fibers remains to be established. Current literature shows that the number of satellite cells is drastically reduced after prolonged denervation (34). Unfortunately, because of limitations in availability of bioptic material, we could not directly address this question in the present work.
In these patients the first mechanical responses in FES-trained muscles can be detected functionally only after a few months of stimulation (24–27), and the force output of these muscles never reaches normal levels (27). This slow recovery is in sharp contrast to the very rapid changes in functional and structural properties occurring in animal experiments with a superimposed pattern of activity or with cross-innervation (35). This time course difference could be explained, in part, by differences in the stimulation programs: in these patients, in fact, daily stimulation was very limited (≈30 min) relative to the conditions usually used in animal experiments (15). Furthermore, in the absence of peripheral nerves, the electrical stimulation is not very effective in activation of muscle fibers.
FES (36, 37) is currently not used to treat paraplegic patients affected by complete lesion of the conus cauda, because standard stimulating devices are not effective in promoting activity in denervated muscles that have undergone severe atrophy. Complete inactivity and immobilization of the limbs, in fact, causes poor blood supply to the denervated areas and a series of secondary complications (osteoporosis, pressure sores, decubital ulcers, etc.), which are determinants of decreases in life expectancy (38, 39). In our patients, restoration of muscle structure and mass is very encouraging. In 4 of 5 patients, muscle force production of the lower extremities under electrical stimulation was sufficiently restored to allow for supported standing up, standing, and even for taking a few steps (see SI Fig. 8 and SI Movies 1 and 2 for more details). The reduction of secondary problems caused by prolonged inactivity, improvement of the patient's quality of life, and possibly their life expectancy, require further investigation.
Materials and Methods
Patient Characteristics.
The 10 subjects (all males) had experienced complete traumatic conus cauda lesion (SCI). All patients were carefully tested to confirm a complete lack of sensory and motor innervation of the quadricep muscles before and after the FES training phase. A detailed description of the functional testing performed (Chronaxie measurements, needle EMG, brain motor control assessment, transcranial and lumbosacral magnetic stimulation) can be found in Modlin et al. (27). Only the patients completely denervated and with no sign of reinnervation were included in the present study. The five control patients (27–37 years of age) had suffered SCI 11 months to 4 years before the biopsy and had not undergone FES treatment (see Table 1). The five FES-treated patients (30–58 years of age) had been denervated for 3.6 years or longer (up to 10.6 years); the FES treatment was for variable periods of time (see Table 2), starting 1.2 to 2.0 years after the injury.
Stimulation Parameters and Functional Response.
Details of the FES regimen are published elsewhere (24, 26, 27). In short, this involves stimulation by surface electrodes, starting with long biphasic stimuli (150- to 300-ms duration, +/−250 mA amplitude, 2 Hz) to elicit muscle twitches in the first phase of training. Later on during training, pulse duration and the interpulse interval are shortened and frequencies of 17–25 Hz are achievable, resulting in fused tetanic contractions.
Hystological and Morphometrical Analysis.
Needle muscle biopsies (1–2 mm diameter, 2–3 mm long, 10–15 mg) were harvested from both right and left vastus lateralis muscles at a single time point for each patient. Cryosections (10 μm thick) were stained as in Kern et al. (25). Morphometric analysis (see SI Fig. 5 A and B) was performed with Scion Image for Windows version Beta 4.0.2 (by 2000 Scion Corporation, Frederick, MD), free software downloaded from the web site (www.scioncorp.com). In the biopsies from SCI patients, 208–250 fibers were measured, whereas in the biopsies from FES-trained patients, 107–216 fibers were measured (Table 1 and 2).
Electron Microscopy.
Needle muscle biopsies (0.5–1 mm diameter, 1–1,5 mm long, 2–5 mg) were harvested from both right and left vastus lateralis muscles at a single time point for each patient. Times elapsed from injury to the biopsy procedure are reported in Tables 1 and 2. Samples for electron microscopy were prepared, sectioned and examined as in Boncompagni et al. (40).
Classification of Muscle Fibers and Measurement of Z Line Width.
Muscle fibers were examined in longitudinal sections, and each muscle fiber was monitored for as long as possible in the section. In the FES-treated patients fibers could be followed for large distances (100–300 μm), whereas in nontreated patients fibers could be usually monitored for shorter distances (usually <100 μm). The fibers were classified according to the level of structural integrity, as described in the Results. Z line width (see SI Fig. 6 and SI Table 3) was measured by using the Soft Imaging System program in micrographs taken at high magnification (×71,000). For each specimen five micrographs were randomly collected from five different fibers. At five random locations along each Z line, Z line width was measured and averaged.
Supplementary Material
Acknowledgments
We thank all of the staff working in the laboratories of Drs. H. Kern, W. Mayr, and Prof. U. Carraro, which with many years of dedicated work allowed the collection of these samples. We also thank Prof. A. Shftiman (Tufts University School of Medicine) for the critical reading of the manuscript and for assistance with English grammar and syntax. This study was supported by Research Grant GGP030289 from the Italian Telethon Foundation and by research funds from the Faculty of Sport Medicine (to F.P.) and by European Union Commission Shared Cost Project RISE Contract QLG5-CT-2001-02191 (to H.K., W.M., and U.C.).
Abbreviations
- EC
excitation-contraction
- FES
functional electrical stimulation
- SCI
spinal cord injury
- SR
sarcoplasmic reticulum
- T tubule
transverse tubule.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709061104/DC1.
References
- 1.Grinnell AD. Physiol Rev. 1995;75:789–834. doi: 10.1152/physrev.1995.75.4.789. [DOI] [PubMed] [Google Scholar]
- 2.Waters RL, Adkins RH, Yakura JS. Paraplegia. 1991;29:573–581. doi: 10.1038/sc.1991.85. [DOI] [PubMed] [Google Scholar]
- 3.Ditunno JF, Little JW, Tessler A, Burns AS. Spinal Cord. 2004;42:383–395. doi: 10.1038/sj.sc.3101603. [DOI] [PubMed] [Google Scholar]
- 4.Pellegrino C, Franzini C. J Cell Biol. 1963;17:327–349. doi: 10.1083/jcb.17.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engel AG, Banker BQT. In: Myology. 3rd Ed. Engel AG, Franzini-Armstrong C, editors. Vol 1. New York: McGraw–Hill; 2004. pp. 749–887. [Google Scholar]
- 6.Lu DX, Huang SK, Carlson BM. Anat Rec. 1997;248:355–365. doi: 10.1002/(SICI)1097-0185(199707)248:3<355::AID-AR8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 7.Condon K, Silberstein L, Blau HM, Thompson WJ. Dev Biol. 1990;138:275–295. doi: 10.1016/0012-1606(90)90197-q. [DOI] [PubMed] [Google Scholar]
- 8.Hoh JF. News Physiol Sci. 1991;6:1–6. doi: 10.1152/physiologyonline.1991.6.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Buller AJ, Eccles JC, Eccles RM. J Physiol (London) 1960;150:399–416. doi: 10.1113/jphysiol.1960.sp006394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eccles JC, Eccles RM, Lundberg A. J Physiol (London) 1958;142:275–291. doi: 10.1113/jphysiol.1958.sp006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edgerton VR, Roy RR, Allen DL, Monti RJ. Am J Phys Med Rehabil. 2002;81:S127–S147. doi: 10.1097/00002060-200211001-00014. [DOI] [PubMed] [Google Scholar]
- 12.Eisenberg BR, Salmons S. Cell Tissue Res. 1981;220:449–471. doi: 10.1007/BF00216750. [DOI] [PubMed] [Google Scholar]
- 13.Lomo T, Westgaard RH, Dahl HA. Proc R Soc Lond B Biol Sci. 1974;187:99–103. doi: 10.1098/rspb.1974.0064. [DOI] [PubMed] [Google Scholar]
- 14.Pette D, Smith ME, Staudte HW, Vrbova G. Pflugers Arch. 1973;338:257–272. doi: 10.1007/BF00587391. [DOI] [PubMed] [Google Scholar]
- 15.Salmons S, Vrbova G. J Physiol (London) 1969;201:535–549. doi: 10.1113/jphysiol.1969.sp008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franzini-Armstrong C, Pincon-Raymond M, Rieger F. Dev Biol. 1991;146:364–376. doi: 10.1016/0012-1606(91)90238-x. [DOI] [PubMed] [Google Scholar]
- 17.Takekura H, Nishi M, Noda T, Takeshima H, Franzini-Armstrong C. Proc Natl Acad Sci USA. 1995;92:3381–3385. doi: 10.1073/pnas.92.8.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vrbova G. J Physiol (London) 1963;169:513–526. doi: 10.1113/jphysiol.1963.sp007276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vrbova G. J Physiol (London) 1966;185:17P–18P. [Google Scholar]
- 20.Gorza L, Gundersen K, Lomo T, Schiaffino S, Westgaard RH. J Physiol (London) 1988;402:627–649. doi: 10.1113/jphysiol.1988.sp017226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanaya F, Tajima T. Clin Orthop Relat Res. 1992:296–301. [PubMed] [Google Scholar]
- 22.Nemoto K, Williams HB, Lough J, Chiu RC. J Reconstr Microsurg. 1988;4:251–255. 257. doi: 10.1055/s-2007-1006928. [DOI] [PubMed] [Google Scholar]
- 23.Schimrigk K, McLaughlin J, Gruninger W. Scand J Rehabil Med. 1977;9:55–60. [PubMed] [Google Scholar]
- 24.Hofer C, Mayr W, Stohr H, Unger E, Kern H. Artif Organs. 2002;26:276–279. doi: 10.1046/j.1525-1594.2002.06951.x. [DOI] [PubMed] [Google Scholar]
- 25.Kern H, Boncompagni S, Rossini K, Mayr W, Fano G, Zanin ME, Podhorska-Okolow M, Protasi F, Carraro U. J Neuropathol Exp Neurol. 2004;63:919–931. doi: 10.1093/jnen/63.9.919. [DOI] [PubMed] [Google Scholar]
- 26.Mayr W, Bijak M, Rafolt D, Sauermann S, Unger E, Lanmuller H. Med Eng Phys. 2001;23:53–60. doi: 10.1016/s1350-4533(01)00014-5. [DOI] [PubMed] [Google Scholar]
- 27.Modlin M, Forstner C, Hofer C, Mayr W, Richter W, Carraro U, Protasi F, Kern H. Artif Organs. 2005;29:203–206. doi: 10.1111/j.1525-1594.2005.29035.x. [DOI] [PubMed] [Google Scholar]
- 28.Eisenberg BR, Kuda AM. J Ultrastruct Res. 1976;54:76–88. doi: 10.1016/s0022-5320(76)80010-x. [DOI] [PubMed] [Google Scholar]
- 29.Sjostrom M, Kidman S, Larsen KH, Angquist KA. J Histochem Cytochem. 1982;30:1–11. doi: 10.1177/30.1.7054271. [DOI] [PubMed] [Google Scholar]
- 30.Bateman JE. Trauma to Nerves in Limbs. Philadelphia: Saunders; 1962. [Google Scholar]
- 31.Sunderland S. Nerves and Nerve Injuries. 2nd Ed. Edinburgh: Churchill Livingstone; 1978. pp. 827–828. [Google Scholar]
- 32.Sanger JW, Sanger JM, Franzini-Armstrong C. In: Myology. 3rd Ed. Engel AG, Franzini-Armstrong C, editors. Vol 1. New York: McGraw–Hill; 2004. pp. 44–65. [Google Scholar]
- 33.Takekura H, Shuman H, Franzini-Armstrong C. J Muscle Res Cell Motil. 1993;14:633–645. doi: 10.1007/BF00141560. [DOI] [PubMed] [Google Scholar]
- 34.Dedkov EI, Kostrominova TY, Borisov AB, Carlson BM. Anat Rec. 2001;263:139–154. doi: 10.1002/ar.1087. [DOI] [PubMed] [Google Scholar]
- 35.Gutmann E, Carlson BM. Pflugers Arch. 1975;353:227–239. doi: 10.1007/BF00584286. [DOI] [PubMed] [Google Scholar]
- 36.Creasey GH, Ho CH, Triolo RJ, Gater DR, DiMarco AF, Bogie KM, Keith MW. J Spinal Cord Med. 2004;27:365–375. doi: 10.1080/10790268.2004.11753774. [DOI] [PubMed] [Google Scholar]
- 37.Peckham PH, Knutson JS. Annu Rev Biomed Eng. 2005;7:327–360. doi: 10.1146/annurev.bioeng.6.040803.140103. [DOI] [PubMed] [Google Scholar]
- 38.DeVivo MJ, Krause JS, Lammertse DP. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- 39.Strauss DJ, Devivo MJ, Paculdo DR, Shavelle RM. Arch Phys Med Rehabil. 2006;87:1079–1085. doi: 10.1016/j.apmr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 40.Boncompagni S, d'Amelio L, Fulle S, Fano G, Protasi F. J Gerontol A Biol Sci Med Sci. 2006;61:995–1008. doi: 10.1093/gerona/61.10.995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.