Abstract
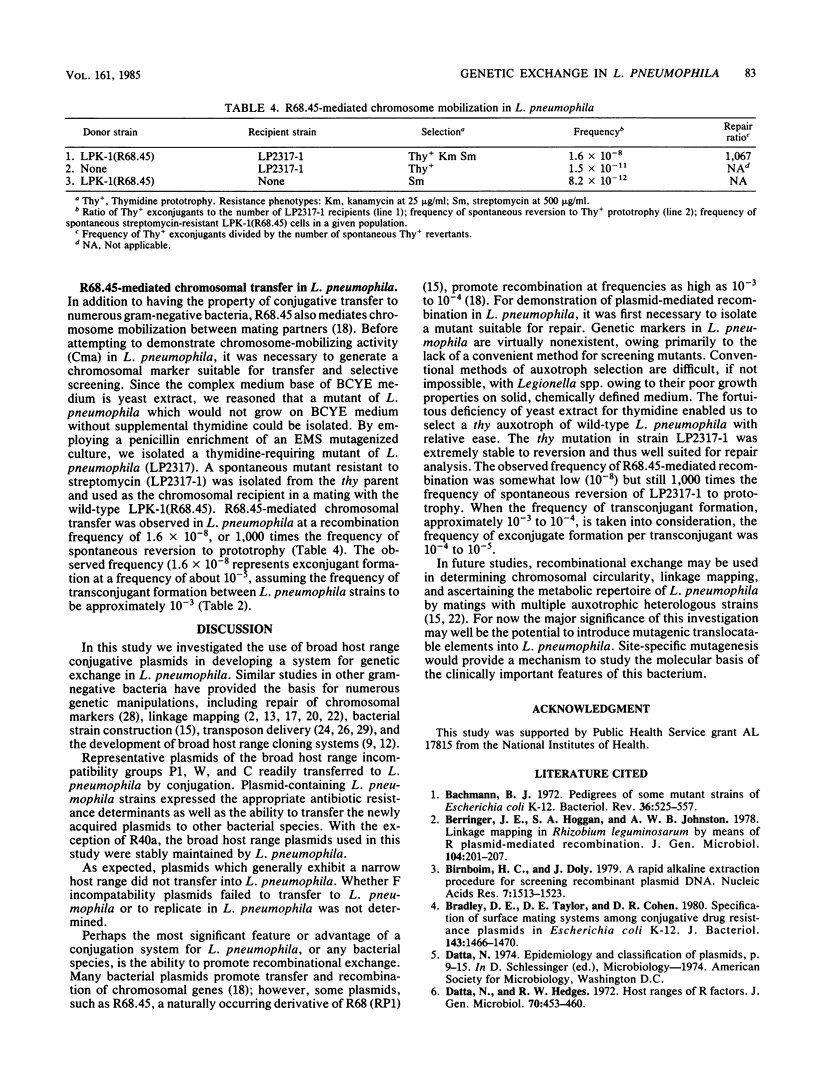
Genetic exchange mechanisms, to our knowledge, have not been reported for Legionella pneumophila, and consequently, studies on the genetic organization of L. pneumophila have not appeared in the literature. Here, we describe gene transfer mediated by broad host range conjugative plasmids in Legionella spp. Escherichia coli strains carrying plasmids RP1 and R68.45 (IncP1), S-a (IncW), and R40a (IncC), but not plasmids of incompatibility groups FI, FII, and FV, served as donors in matings with L. pneumophila Knoxville 1 (LPK-1). Transconjugants selected by resistance to kanamycin (RP1, R68.45, and S-a) and carbenicillin (R40a) were observed at frequencies of 6.6 X 10(-3), 4.7 X 10(-3), 2.2 X 10(-4), and 5.4 X 10(-5), respectively. Plasmid transfer was not affected by DNase added to the mating medium. After plasmid transfer, LPK-1 stably maintained RP1, R68.45, and S-a, but not R40a. Plasmid-containing LPK-1 isolates also served as donors in agar plate matings with E. coli W1485-1 and naladixic acid-resistant mutants of LPK-1, Legionella micdadei, and Legionella longbeachii. Recombinational exchange of a chromosomal trait was demonstrated when a thymidine auxotroph of L. pneumophila was repaired by R68.45-mediated chromosomal mobilization of a prototrophic donor strain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E., Taylor D. E., Cohen D. R. Specification of surface mating systems among conjugative drug resistance plasmids in Escherichia coli K-12. J Bacteriol. 1980 Sep;143(3):1466–1470. doi: 10.1128/jb.143.3.1466-1470.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Host ranges of R factors. J Gen Microbiol. 1972 May;70(3):453–460. doi: 10.1099/00221287-70-3-453. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. R factors identified in Paris, some conferring gentamicin resistance, constitute a new compatibility group. Ann Inst Pasteur (Paris) 1972 Dec;123(6):849–852. [PubMed] [Google Scholar]
- Dennison S. Naturally occurring R factor, derepressed for pilus synthesis, belonging to the same compatibility group as the sex factor F of Escherichia coli K-12. J Bacteriol. 1972 Jan;109(1):416–422. doi: 10.1128/jb.109.1.416-422.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein P. H. Comparative study of selective media for isolation of Legionella pneumophila from potable water. J Clin Microbiol. 1982 Oct;16(4):697–699. doi: 10.1128/jcm.16.4.697-699.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. W., Tsai T. R., Orenstein W., Parkin W. E., Beecham H. J., Sharrar R. G., Harris J., Mallison G. F., Martin S. M., McDade J. E. Legionnaires' disease: description of an epidemic of pneumonia. N Engl J Med. 1977 Dec 1;297(22):1189–1197. doi: 10.1056/NEJM197712012972201. [DOI] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Fulbright D. W., Leary J. V. Linkage analysis of Pseudomonas glycinea. J Bacteriol. 1978 Nov;136(2):497–500. doi: 10.1128/jb.136.2.497-500.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted J., Saunders J. R., Ingram L. C., Sykes R. B., Richmond M. H. Properties of a R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972 May;110(2):529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D., Holloway B. W. Chromosome mobilization by the R plasmid R68.45: a tool in Pseudomonas genetics. Mol Gen Genet. 1978 Jan 17;158(3):229–237. doi: 10.1007/BF00267194. [DOI] [PubMed] [Google Scholar]
- Holloway B. W. Isolation and characterization of an R' plasmid in Pseudomonas aeruginosa. J Bacteriol. 1978 Mar;133(3):1078–1082. doi: 10.1128/jb.133.3.1078-1082.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W. Plasmids that mobilize bacterial chromosome. Plasmid. 1979 Jan;2(1):1–19. doi: 10.1016/0147-619x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- McDade J. E., Shepard C. C., Fraser D. W., Tsai T. R., Redus M. A., Dowdle W. R. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977 Dec 1;297(22):1197–1203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- Megias M., Caviedes M. A., Palomares A. J., Perezsilva J. Use of plasmid R68.45 for constructing a circular linkage map of the Rhizobium trifolii chromosome. J Bacteriol. 1982 Jan;149(1):59–64. doi: 10.1128/jb.149.1.59-64.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. D. Legionella infections: a review of five years of research. Rev Infect Dis. 1983 Mar-Apr;5(2):258–278. doi: 10.1093/clinids/5.2.258. [DOI] [PubMed] [Google Scholar]
- Morgan A. F. Isolation and characterization of Pseudomonas aeruginosa R' plasmids constructed by interspecific mating. J Bacteriol. 1982 Feb;149(2):654–661. doi: 10.1128/jb.149.2.654-661.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neill E. A., Berlinberg C., Bender R. A. Activity of Plasmid Replicons in CAULOBACTER CRESCENTUS : Rp4 and Cole1. Genetics. 1983 Apr;103(4):593–604. doi: 10.1093/genetics/103.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. H., Shipley P. Host range and properties of the Pseudomonas aeruginosa R factor R1822. J Bacteriol. 1973 Feb;113(2):772–780. doi: 10.1128/jb.113.2.772-780.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Sato M., Staskawicz B. J., Panopoulos N. J., Peters S., Honma M. A host-dependent hybrid plasmid suitable as a suicidal carrier for transposable elements. Plasmid. 1981 Nov;6(3):325–331. doi: 10.1016/0147-619x(81)90040-8. [DOI] [PubMed] [Google Scholar]
- Towner K. J., Vivian A. RP4-mediated conjugation in Acinetobacter calcoaceticus. J Gen Microbiol. 1976 Apr;93(2):355–360. doi: 10.1099/00221287-93-2-355. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]