Abstract
Polycomb group genes (PcGs) have been implicated in cancer based on altered levels of expression observed in certain tumors and the behavior of cultured cells containing inserted PcG transgenes. Endometrial stromal tumors provide evidence for a direct causal relationship because they contain several chromosomal translocations and resultant gene fusions involving PcGs, the most common of which joins portions of theJAZF1 gene to the PcGJJAZ1/SUZ12. We show here that both benign and malignant forms of this tumor have theJAZF1–JJAZ1 fusion but only the malignant form also exhibits exclusion of the unrearrangedJJAZ1 allele. To evaluate the effects of both theJJAZ1/SUZ12 fusion and allelic exclusion on functions related to cell growth, we studied HEK293 cells that were modified with respect toJJAZ1 expression. We found that theJAZF1–JJAZ1 fusion restored levels of the polycomb protein EZH2 and histone 3 lysine 27 trimethylation, which were reduced by knockdown of endogenous JJAZ1. At the same time, the presence ofJAZF1–JJAZ1 markedly inhibited apoptosis and induced above normal proliferation rates, although the latter effect occurred only when normalJJAZ1 was suppressed. Our findings suggest a genetic pathway for progression of a benign precursor to a sarcoma involving increased cell survival associated with acquisition of a PcG rearrangement, followed by accelerated cellular proliferation upon allelic exclusion of the unrearranged copy of that gene. Furthermore, these results indicate the likely functional importance of allelic exclusion of genes disrupted by chromosomal translocations, as seen in a variety of other cancers.
Keywords: chromosomal translocation, endometrial stromal tumor, polycomb group gene, chromatin remodeling, apoptosis
Polycomb group genes (PcGs) encode proteins that act in multimeric complexes to methylate histones, leading to chromatin remodeling, regional compaction of the chromatin, and suppression of transcription from genes associated with these regions (1, 2). Increased levels of polycomb proteins have been detected in a variety of human cancers (3, 4), often correlated with more aggressive clinical behavior (5, 6). Aberrant coexpression of various PcGs has been noted in the malignant cells of certain cancers, and neoplastic transformation of cells in culture has been achieved by over-expression of PcGs from inserted cDNA sequences (7). Although such observations imply a role for PcGs in the transformed phenotype of neoplastic cells, the proximity of abnormal levels of Polycomb proteins to the primary changes in the cell that control neoplastic behavior is unclear.
Endometrial stromal tumors provide evidence for a primary role of PcGs in neoplastic cell behavior because these tumors contain a series of rearrangements of DNA within several PcGs. These tumors arise in the mesenchymal tissue that underlies the surface epithelium of the uterine cavity and surrounds endometrial glands formed by invaginations of the surface epithelium. Three major types of tumors derive from this tissue: endometrial stromal nodules (SNs), endometrial stromal sarcomas (ESSs), and undifferentiated uterine sarcomas (8–10). Whereas the latter tumor shows highly atypical cytological features, the other two tumors are both composed of cells that histologically resemble the cells of normal endometrial stroma during the proliferative phase of the menstrual cycle (8–10). The principal difference between these two tumors is that SNs do not show invasion of surrounding tissues within the uterus and are therefore considered benign. In contrast, ESSs show invasion and are malignant.
By using cytogenetics and molecular methods of detection, approximately one-half of ESSs have been shown to contain a specific recurrent chromosomal translocation, the t(7;17)(p15;q21) (11–15). More recently, this translocation has been found in each case of SNs analyzed, although the total number of such tumors tested is necessarily small because of the rarity of these neoplasms (12). Recombination of DNA at the site of breakage and rejoining in the translocation results in the fusion of two genes, JAZF1 and JJAZ1, and production of a chimeric RNA in which the first three exons (from five total) of JAZF1 are joined to the last 15 (from 16) of JJAZ1 (11). Neither the reciprocal RNA nor its protein product has been identified within ESSs.
Little is known about the function of JAZF1, although there is one report of coimmunoprecipitation of the JAZF1 protein with the TAK1 orphan nuclear receptor (16). On the other hand, several years after the discovery of JJAZ1 through its alteration by the t(7;17), its ortholog in Drosophila was identified to be the gene mutated in flies with the SuZ12 phenotype (17). Since then, the JJAZ1/SUZ12 protein has been shown to be an essential component, along with the proteins EZH2 and EED, of the polycomb repression complex 2 (PRC2) (18, 19), which is responsible for the methylation of lysines 9 and 27 in histone 3 (20–28).
The mechanism by which altered JJAZ1 presumably contributes to the neoplastic phenotype in SNs and ESSs is not known, nor is it understood what distinguishes the malignant behavior of ESSs from the benign behavior of SNs. Here we report that expression of the unrearranged allele of JJAZ1 in ESSs with the t(7;17) is suppressed, as opposed to the unrearranged allele in SNs, in which it is fully active. Difficulties in the stable insertion of transgenes into cultured endometrial stromal cells have hampered study of JJAZ1 alterations in the normal counterpart to cells of ESS; however, introduction of a JAZF1–JJAZ1 expression vector into cultured 293 cells produces a number of significant effects on the growth properties of these cells. Besides restoring to near normal levels the reduced amounts of EZH2 and total trimethyl histone 3 lysine 27 (H3K27) seen when JJAZ1 is deficient in these cells, introduction of a JAZF1–JJAZ1 vector into cells promotes proliferation of these cells. This effect depends on suppression of the endogenous JJAZ1, because in the presence of normal JJAZ1 expression, JAZF1–JJAZ1 decreases proliferation. However, expression of JAZF1–JJAZ1 with or without suppression of JJAZ1 protects cells from serum deprivation and hypoxia-induced apoptosis.
Results
Suppression of the UnrearrangedJJAZ1 Allele in ESSs with the t(7;17).
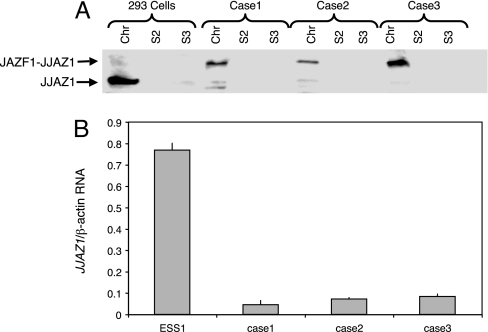
Expression of JJAZ1 was analyzed by Western blots, which was performed with anti-JJAZ1 antibody generated by us against JJAZ1 polypeptides. A higher band, corresponding to the predicted molecular weight of the amino-terminal JAZF1 to carboxyl-terminal JJAZ1 chimeric protein, was observed in the three primary tumor cell lines derived from ESSs. These three tumors have been demonstrated to harbor the t(7;17) translocation by conventional cytogenetics, fluorescence in situ hybridization (FISH), and RT-PCR amplification of RNA sequences across the site of fusion in the JAZF1–JJAZ1 transcription product. Additionally, normal JJAZ1 was almost undetectable in these three cases (Fig. 1A). To confirm this finding and check whether suppression of JJAZ1 occurs at a pretranslational level, we performed RT-PCR with primers designed to amplify only the WT allele of JJAZ1 by annealing to sequences of JJAZ1 RNA (and its complement) 5′ of the site of fusion of JAZF1 and JJAZ1 in the chimeric transcript. Fig. 1B demonstrates at least a 10-fold decrease of the WT JJAZ1 transcript in tumor cells compared with an ESS cell line, ESS1, which lacks the t(7;17) translocation.
Fig. 1.
Analysis of JJAZ1 protein and mRNA in ESS cell lines. (A) Western blot of cell lines analyzed with anti-JJAZ1 antiserum. Cases 1, 2, and 3 are primary ESS cell lines. Chr, S2, and S3 represent the chromatin-enriched cell fraction, the soluble cytoplasmic fraction, and the soluble nuclear fraction, respectively. (B) Real-time RT-PCR for JJAZ1 mRNA. The amount of WT allele JJAZ1 mRNA was compared with the amount of β-actin mRNA. ESS1 is an ESS cell line derived from a tumor lacking the t(7;17).
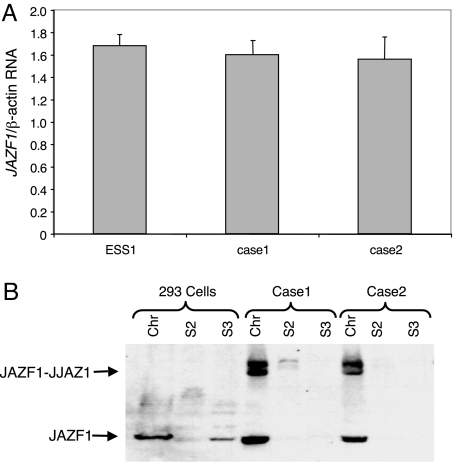
To test whether the WT allele of JAZF1 is also subjected to suppression in ESSs, we performed an analogous analysis for transcription of this gene, by using RT-PCR with primers designed to amplify only sequences of JAZF1 3′ of the site of fusion in the chimeric transcript. No difference was observed between the two t(7;17) ESS cases and the ESS1 cell line (Fig. 2A). Furthermore, Western blot using anti-JAZF1 antibody that was prepared by us detected similar level of WT JAZF1 in primary ESS cell lines, along with JAZF1–JJAZ1 chimeric protein (Fig. 2B).
Fig. 2.
Analysis of JAZF1 protein and mRNA in ESS cell lines. (A) Real-time RT-PCR for JAZF1 mRNA. The amount of WT JJAZ1 mRNA was normalized against the amount of β-actin mRNA. (B) Western blot of cell lines analyzed with anti-JAZF1 antiserum. Cases 1 and 2 are primary ESS cell lines.
Absence of WTJJAZ1 Suppression in SNs.
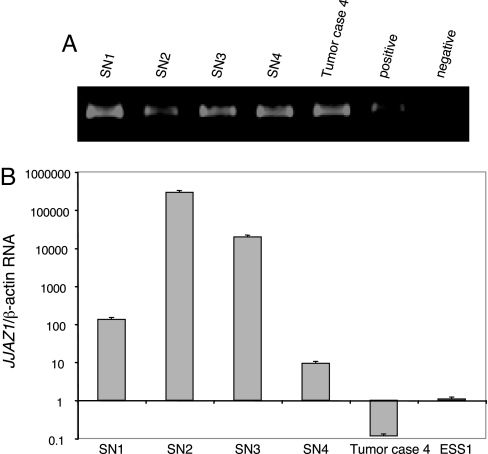
All cases of SNs available to us showed evidence of the t(7;17) by RT-PCR of JAZF1–JJAZ1 RNA and by FISH (Fig. 3A). Quantitative RT-PCR was performed to assess the activity of the WT JJAZ1 gene. Contrary to ESSs, all nodules tested contained high levels of WT JJAZ1 RNA (Fig. 3B).
Fig. 3.
Analysis of mRNA in SN cases. (A) RT-PCR for JAZF1–JJAZ1 fusion mRNA in SN cases 1–4 and a t(7;17)-positive tumor as a positive control. (B) Real-time RT-PCR for JJAZ1 mRNA in four SN cases as well as the t(7;17)-positive tumor case 4.
Reduced EZH2 and H3K27 Trimethylation and Proliferation in 293 Cells After Suppression of JJAZ1 Expression.
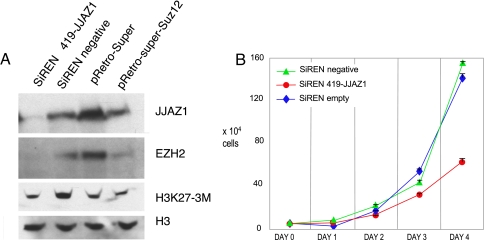
We attempted to study the effect of the JAZF1–JJAZ1 fusion in a cell culture model simulating the in vivo situation in ESSs by insertion of expression vectors into cultured normal human endometrial stromal cells. These experiments were unsuccessful because of failure to insert exogenous sequences into these cells with retroviral vectors. Although initial infection proved possible, transduced sequences were rapidly eliminated from the cells. Moreover, numerous attempts at transfecting these cells by using a variety of agents to promote uptake of DNA plasmids did not yield cells in which transfected DNA was stably integrated into the host cell genomes. Consequently, we conducted further experiments by using 293 cells. Specifically, by using these cells, we sought to determine the influence of the JAZF1–JJAZ1 fusion in the presence and absence of endogenous JJAZ1 expression. We began by reducing the level of WT JJAZ1 RNA by siRNA molecules that target the JJAZ1 mRNA 5′ to the site of fusion with JAZF1 in JAZF1–JJAZ1 mRNA, such that expression from JAZF1–JJAZ1 expression vectors later introduced into the cells should not be affected. Cells stably infected with the retrovirus carrying the siRNA construct and a puromycin resistance marker were selected by growth in antibiotic and collected for Western blot analysis (Fig. 4A). Compared with the negative control, consisting of 293 cells infected with a vector containing a siRNA that does not recognize any known human mRNA, cells with the JJAZ1 siRNA vector showed knockdown of JJAZ1 protein, reduced EZH2 protein, and discernibly lower levels of H3K27 trimethylation. Similar results were also obtained with another siRNA construct targeting 3′ of JJAZ1, as previously described (23).
Fig. 4.
Effects of siRNA knockdown of JJAZ1 in 293 cells. (A) Western blot analyses of protein extracts from 293 cells stably infected with an pSiREN vector encoding siRNA targeted to JJAZ1 mRNA sequence 5′ to the fusion point (419-JJAZ1); a negative control siRNA that does not recognize any known human mRNA; an empty vector control of another siRNA vector system (pRetro-Super); and pRetro-Super containing an siRNA targeting JJAZ1 mRNA 3′ of the fusion point (pRetro-SuperSuz12). Histone 3 served as loading control. Compared with negative control or empty vector control, siRNA targets 5′ or 3′ of JJAZ1 substantially reduced JJAZ1, secondarily reduced EZH2 because of the presumed destabilization in the absence of JJAZ1, and modestly reduced trimethyl H3K27, as reported by others (21, 23). (B) Effects of JJAZ1 knockdown on 293 cell proliferation. Compared with negative control siRNA or empty vector, knocking down JJAZ1 slowed 293 cell growth. The y axis is calibrated in total cells, and the x axis is calibrated in days of growth. Cell numbers at each time point were determined by suspending cells in Hank's buffered saline after trypsinizing the cells growing in monolayers and counting suspended cells in a hemocytometer. The results shown were from experiments done in triplicate. Three additional experiments that were performed in triplicate yielded similar results.
Additionally, 293 cells in which JJAZ1 was knocked down exhibited a slower growth rate (Fig. 4B). A reduced rate of growth was observed whether knockdown was achieved with either 5′ or 3′ siRNA. Negative control siRNA vectors and infections using the empty vector showed no difference in growth rate.
Restoration of EZH2 Levels and H3K27 Trimethylation by theJAZF1–JJAZ1 Fusion.
JAZF1–JJAZ1 and JJAZ1 cDNA sequences were ligated behind the CMV promoter and just before the internal ribosomal entry site (IRES)–GFP sequence in the MIGRI–GFP retroviral vector. After infection with the retrovirus, 293 cells fluorescent for GFP were collected and Western blot analysis was performed on protein extracts from the cells. Fig. 5A shows a restored level of EZH2 when JAZF1–JJAZ1 was introduced into 293 cells in which JJAZ1 mRNA had been knocked down previously with siRNA. H3K27 trimethylation was returned to near normal levels in these cells as well.
Fig. 5.
Effects of JJAZ1 and JAZF1–JJAZ1 transgenes on 293 cells with and without knock down of endogenous JJAZl expression. (A) 293 cells that were infected previously with virus encoding siRNA targeting JJAZ1 mRNA 5′ of the fusion point (419-JJAZ1) were infected again with viral vectors expressing JJAZ1 (MIGRI-JJAZ1), JAZF1–JJAZ1 (MIGRI-J-J), or no insert (MIGRI-empty). Histone 3 served as loading control. Compared with empty vector control (MIGRI-empty), JAZF1–JJAZ1 reestablished EZH2 and H3K27 trimethylation to a level similar to that in parental 293 cells. (B) Effect of JAZF1–JJAZ1 on 293 cell proliferation after siRNA knockdown of JJAZ1 expression. Introducing JAZF1–JJAZ1 induced faster growth rate than parental 293 cell. (C) Effect of JAZF1–JJAZ1 on 293 cell proliferation without siRNA knockdown of JJAZ1 expression. JAZF1–JJAZ1 expression reduced the cell proliferation rate compared with empty vector control.
Increased Proliferation of 293 Cells Induced by theJAZF1–JJAZ1 Fusion When Endogenous, WT JJAZ1 Is Suppressed.
Assays of cell proliferation showed that stable infection with a JJAZ1 expression vector only partially reversed the decreased growth rate caused by the 419-JJAZ1 siRNA compared with the proliferative rate of parental 293 cells, presumably because of the siRNA interference with expression of the JJAZ1 transgene in these cells (Fig. 5A). When JAZF1–JJAZ1 was introduced into these cells, a significantly faster growth rate was noted, even faster than observed with the original 293 cells (P = 0.003) (Fig. 5B). However, this faster rate of proliferation was only achieved in the cells containing 419-JJAZ1 siRNA. In negative control cells lacking siRNA, JAZF1–JJAZ1 did not promote growth but rather made cells grow even more slowly (P = 0.013) (Fig. 5C).
Increased Survival in Serum-Deprived and Hypoxic Cultures of Cells Expressing theJAZF1–JJAZ1 Fusion.
To study the effects of JJAZ1 knockdown and expression of JAZF1–JJAZ1 on cell survival and apoptosis, we cultured our genetically modified 293 cells under two conditions toxic to cells: serum starvation and hypoxia simulated by addition of desferrioxamine to the culture medium (29, 30). In both cases, knockdown of JJAZ1 increased somewhat the number of surviving cells after incubation of the cells under conditions associated with decreased cell viability (Figs. 6 and 7A). The addition of JAZF1–JJAZ1 to the cells enhanced survival of cells significantly more than did knockdown of JJAZ1.
Fig. 6.
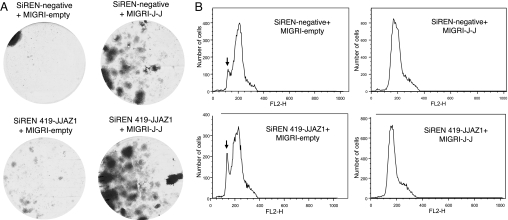
Cell survival after culture in serum-deprived media. (A) Total numbers of surviving cells per dish according to the protocol described under Materials and Methods, after initial seeding with 104 cells. (B) Images of representative tissue culture dishes showing numbers of colonies after staining with crystal violet. Dishes were initially seeded with 2 × 103 cells. The number adjacent to each dish indicates the total individual colonies counted in that dish under a low magnification. (C) Histogram of the numbers of colonies counted in experiments described for B.
Fig. 7.
Cell survival after culture under hypoxic conditions simulated by addition of desferrioxamine, as described under Materials and Methods. (A) Images of representative tissue culture dishes showing numbers of colonies after staining with crystal violet. Dishes were initially seeded with 2 × 103 cells. Surviving colonies containing the JAZF1–JJAZ1 fusion tended to be confluent by the time any colonies in the control dishes appeared, thereby precluding accurate quantitative assessment of the survival advantage to cells with the JAZF1–JJAZ1 fusion, as in Fig. 6C. (B) FACS analysis of DNA content in cells stained with propidium iodide. The vertical arrows indicate the peak of sub2n cells, reflecting ongoing apoptosis in these cells. The horizontal axis represents the number of cells.
Increased cell survival under adverse culture conditions generally appears to be associated with depressed apoptosis, which we specifically assessed in modified 293 cells by the DNA loss from cells in medium supplemented with desferrioxamine (Fig. 7B). Knockdown of JJAZ1 had no discernable effect on the number of cells containing sub2n amounts of DNA, which provide an index of active apoptosis within the population, whereas expression of JAZF1–JJAZ1 virtually returned the number of cells with sub2n DNA to that of cells grown under standard culture conditions. This reduction in cells with sub2n DNA was observed independently of JJAZ1 expression levels.
Discussion
The experiments described here were modeled on the changes in the structure and activity of the JJAZ1 gene observed in endometrial stromal tumors; however, 293 cells were used for these studies because of the inability to achieve stable expression of exogenous genes introduced into either cultured normal endometrial stromal cells or immortalized sublines produced from these cultures. Inferences about the effects of altered JJAZ1 functions in endometrial stromal tumors based on our experiments should be interpreted in light of the fact that we did not use endometrial stromal cells in our studies. In this respect, our studies are not unlike those using murine NIH 3T3 fibroblast cells to assay for oncogenic activity of mutant genes found in human epithelial tumors (31–34). However, ESS cells and 293 cells are both mesenchymal in origin and derived from human tissues.
Aside from the experimental results obtained in our studies, the finding of the same specific chromosomal translocation in both ESSs and SNs suggests a common genetic pathway for these two tumors, the ultimate relationship being that SNs represent precursors of ESSs. Formerly, these tumors generally were not thought to be etiologically related. In fact, benign precursors of sarcomas have not been described previously, as opposed to the various benign lesions known to be precursors of carcinomas (e.g., adenomatous polyps and colon cancer, pigmented congenital nevi and melanomas). Despite the current prevailing view that SNs and ESSs develop independently, there are numerous anecdotal reports of tumors diagnosed originally as SNs followed years later by metastatic ESSs. Whereas the original diagnoses in such cases may simply have been incorrect, our data provide an alternative, biologic explanation to support these observations.
If endometrial SNs are precursor lesions for ESSs, there are likely additional genetic and epigenetic changes acquired by cells in SNs that drive transformation to the sarcomatous state. Analysis of the JJAZ1 expression in the relatively small set of ESSs available to us (in large part attributable to the rarity of the tumor) suggests that suppression of the unrearranged JJAZ1 allele is a candidate for one such change. Although constrained by the use of nonendometrial stromal cells for our studies, the observation that the JAZF1–JJAZ1 fusion enhances cell proliferation only in the presence of silenced endogenous JJAZ1 alleles of cultured 293 cells would seem consistent with this interpretation. In this respect, JJAZ1 appears to have features of both an oncogene and a tumor suppressor gene, in that fusion with JAZF1 acts as a dominant mutation, as seen with other genes altered by chromosomal translocations in cancer; however, the second allele must be inactivated for full malignancy, as seen with tumor suppressor genes.
Whereas the JAZF1–JJAZ1 fusion in 293 cells with knocked down JJAZ1 expression stimulates cellular proliferation, the JAZF1–JJAZ1 fusion in cells with active JJAZ1 genes is growth-inhibitory. This result was initially surprising to us, given that SNs, all of which contain the JAZF1–JJAZ1 fusion, are tumors (albeit benign ones) and therefore composed of cells that should also have some growth advantage relative to normal endometrial stromal cells. However, our results on the prosurvival properties of the JAZF1–JJAZ1 fusion on cells grown under the stress of serum deprivation or hypoxia may explain this finding. Possibly, SN cells divide more slowly than those of ESSs or even normal cells but because of the presence of the JAZF1–JJAZ1 fusion are more resistant to loss of growth factors in serum or to hypoxia. Resistance of neoplastic cells under conditions of reduced serum and hypoxia is probably generally important in tumors, which often have a precarious blood supply, especially among tumors arising within the premenopausal endometrium, which undergoes vascular collapse and extensive apoptosis on a monthly basis.
The feature of ESSs that is not accounted for by the JAZF1–JJAZ1 fusion and allelic exclusion of JJAZ1 in our model tissue culture system is tissue invasion. Experiments designed to test for this property among 293 cells with knocked down JJAZ1 and insertion of the JAZF1–JJAZ1 fusion failed to demonstrate increased invasiveness of these cells in Boyden chambers, a frequently used model system used to study the invasiveness of cells (data not shown). Presumably, other alterations in the cells making up endometrial stromal tumors are necessary to endow cells with the ability to invade surrounding tissues and to metastasize. A second possibility is that the Boyden chamber is simply limited at detecting invasive properties of all cell types capable of spread and dissemination in vivo.
It remains to be determined whether allelic exclusion has effects similar to those associated with the JAZF1–JJAZ1 fusion in tumors having characteristic chromosomal aberrations involving genes other than JAZF1 or JJAZ1. The basic phenomenon of allelic exclusion is not rare in such tumors. For example, the unrearranged allele of BCL2 is transcriptionally silenced in non-Hodgkin's follicular B cell lymphomas carrying the t(14;18)(q32;q21) (35); the MYCC allele on the normal chromosome 8 in most cases of Burkitt's lymphoma bearing the t(8;14)(q24;q32) or variant translocations is transcriptionally partly or completely inactive (36, 37); and in chronic myelocytic leukemia, the ABL allele unaltered by the t(9;22)(q34;q11) (or the Philadelphia chromosome) is transcribed but then shuts down during transition to blast crisis (38).
The cause of suppression of the unrearranged JJAZ1 allele in ESSs is not presently understood. Nevertheless, certain processes can be ruled out. Among these is constitutive allelic exclusion established during early normal development, as seen in olfactory receptor genes and several cytokine genes (39–41), because the unrearranged JJAZ1 allele is expressed in SNs containing the t(7;17). Additionally, we have directly demonstrated expression of both alleles distinguished by polymorphisms in coding sequences in nonendometrial cells (data not shown). Another theoretical mechanism for allelic exclusion that appears untrue is deletion of DNA within ESSs, because both FISH and Southern blot analyses of JJAZ1 DNA showed no apparent abnormality. Furthermore, the products of the JAZF1–JJAZ1 fusion do not suppress expression of the unrearranged JJAZ1 allele because (i) that allele is active in SNs and (ii) expression of JAZF1–JJAZ1 cDNA in cells transfected with expression vectors does not affect activity of the endogenous JJAZ1 alleles. The possibility remains that allelic expression of the unrearranged JJAZ1 allele is a stochastic, epigenetic phenomenon, which, in combination with the JAZF1–JJAZ1 fusion, provides a proliferative advantage to cells containing those alterations.
Decreased levels of EZH2 and H3K27 trimethylation caused by suppression of JJAZ1 are consistent with previous reports, presumably because JJAZ1 stabilizes EZH2 protein (21, 23). Slower growth rate was also observed with knocked down JJAZ1, as described before (23). When JAZF1–JJAZ1 fusion was introduced into these cells, EZH2 and H3K27 trimethylation levels were largely restored, suggesting the fusion retains this basic function of JJAZ1 and does not affect the overall histone methylation levels. Whether the fusion affects specific targets of the PRC2 complex, and thus changes chromatin structure at particular loci, is currently unknown.
All of the SNs we have examined harbor the JAZF1–JJAZ1 fusion, but only approximately half of ESSs have the fusion. However, approximately half of those ESSs lacking the JAZF1–JJAZ1 fusion (a total of 25%) are reported to contain a fusion of the 5′ end of JAZF1 with the 3′ end of another PcG, PHF1. In the one case of an ESS that had a JAZF1-PHF1 fusion and was available to us for study, the unrearranged PHF1 was transcriptionally silenced (data not shown), similar to the situation with JJAZ1 in other ESSs. These observations suggest a general pathway for development of ESSs that involves acquisition of a fusion of JAZF1 with a PcG followed by silencing of the unarranged allele of that same gene.
Materials and Methods
Tissues and Cultured Cells.
All of the tissues of ESS and nodules were collected in either Brigham Woman's Hospital or Yale–New Haven Hospital, according to Health Insurance Portability and Accountability Act regulations governing the use of human tissues. 293 cells were maintained in RPMI medium 1640 containing 10% FBS. Derivation of stable cells, cell growth assay, cell survival assay and apoptotic assay were performed with standard procedure as detailed in supporting information (SI) Materials and Methods.
Retroviral Vector Constructs.
Sense and antisense oligonucleotides for SiRNA targeting JJAZ1 were annealed and ligated into vectors by using the pSIREN-RetroQ system (Clontech). Expression vectors for JJAZ1 or the JAZF1–JJAZ1 fusion were constructed by inserting the full-length coding region of the genes in front of the IRES in the MIGRI–GFP retroviral plasmid (42). Retroviral supernatants were obtained by using standard procedure as detailed in SI Materials and Methods.
RT-PCR and Real-Time PCR.
RNA was extracted by using TRIzol reagent (Invitrogen). cDNA was generated with AMV-RT kit (Roche) by using random hexamer priming. Real-time PCR was performed with standard methods as detailed in SI Materials and Methods.
Antisera and Antibodies.
Peptide antibodies against JJAZ1 and JAZF1 were generated as detailed in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Ed-Chun Chen for help with propidium iodide staining and FACS analysis and Dr. K. Helin (Biotech Research & Innovation Centre, Copenhagen, Denmark) for kindly providing the pRetro-Super and pRetro-SuperSuz12 retroviral construct. We thank Dr. Zenta Walther for critical reading and help with preparation of the manuscript. This work was supported by National Cancer Institute Grant R01 CA85995.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709986104/DC1.
References
- 1.Strahl BD, Allis CD. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 2.Jenuwein T, Allis CD. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez V, Hartmann E, Ott G, Campo E, Rosenwald A. J Clin Oncol. 2005;23:6364–6369. doi: 10.1200/JCO.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Bea S, Tort F, Pinyol M, Puig X, Hernandez L, Hernandez S, Fernandez PL, van Lohuizen M, Colomer D, Campo E. Cancer Res. 2001;61:2409–2412. [PubMed] [Google Scholar]
- 5.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, et al. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 6.Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, et al. Proc Natl Acad Sci USA. 2003;100:11606–11611. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haupt Y, Bath ML, Harris AW, Adams JM. Oncogene. 1993;8:3161–3164. [PubMed] [Google Scholar]
- 8.Chang KL, Crabtree GS, Lim-Tan SK, Kempson RL, Hendrickson MR. Am J Surg Pathol. 1990;14:415–438. doi: 10.1097/00000478-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Chang KL, Crabtree GS, Lim-Tan SK, Kempson RL, Hendrickson MR. Int J Gynecol Pathol. 1993;12:282–296. [PubMed] [Google Scholar]
- 10.Kurman RJ. Blaustein's Pathology of the Female Genital Tract. New York: Springer; 2002. [Google Scholar]
- 11.Koontz JI, Soreng AL, Nucci M, Kuo FC, Pauwels P, van Den Berghe H, Cin PD, Fletcher JA, Sklar J. Proc Natl Acad Sci USA. 2001;98:6348–6353. doi: 10.1073/pnas.101132598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nucci MR, Harburger D, Koontz J, Cin PD, Sklar J. Am J Surg Pathol. 2007;31:65–70. doi: 10.1097/01.pas.0000213327.86992.d1. [DOI] [PubMed] [Google Scholar]
- 13.Dal Cin P, Aly MS, De Wever I, Moerman P, Van Den Berghe H. Cancer Genet Cytogenet. 1992;63:43–46. doi: 10.1016/0165-4608(92)90062-d. [DOI] [PubMed] [Google Scholar]
- 14.Hennig Y, Caselitz J, Bartnitzke S, Bullerdiek J. Cancer Genet Cytogenet. 1997;98:84–86. doi: 10.1016/s0165-4608(96)00393-7. [DOI] [PubMed] [Google Scholar]
- 15.Pauwels P, Dal Cin P, Van de Moosdijk CN, Vrints L, Sciot R, Van den Berghe H. Histopathology. 1996;29:84–87. [PubMed] [Google Scholar]
- 16.Nakajima T, Fujino S, Nakanishi G, Kim YS, Jetten AM. Nucleic Acids Res. 2004;32:4194–4204. doi: 10.1093/nar/gkh741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birve A, Sengupta AK, Beuchle D, Larsson J, Kennison JA, Rasmuson-Lestander A, Muller J. Development. 2001;128:3371–3379. doi: 10.1242/dev.128.17.3371. [DOI] [PubMed] [Google Scholar]
- 18.Levine SS, King IF, Kingston RE. Trends Biochem Sci. 2004;29:478–485. doi: 10.1016/j.tibs.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Lund AH, van Lohuizen M. Curr Opin Cell Biol. 2004;16:239–246. doi: 10.1016/j.ceb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 21.Cao R, Zhang Y. Mol Cell. 2004;15:57–67. doi: 10.1016/j.molcel.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Cao R, Zhang Y. Curr Opin Genet Dev. 2004;14:155–164. doi: 10.1016/j.gde.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Pasini D, Bracken AP, Jensen MR, Lazzerini Denchi E, Helin K. EMBO J. 2004;23:4061–4071. doi: 10.1038/sj.emboj.7600402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sparmann A, van Lohuizen M. Nat Rev Cancer. 2006;6:846–856. doi: 10.1038/nrc1991. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz YB, Pirrotta V. Nat Rev Genet. 2007;8:9–22. doi: 10.1038/nrg1981. [DOI] [PubMed] [Google Scholar]
- 26.Kuzmichev A, Nishioka K, Erdjument-Bromage H, Tempst P, Reinberg D. Genes Dev. 2002;16:2893–2905. doi: 10.1101/gad.1035902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V. Cell. 2002;111:185–196. doi: 10.1016/s0092-8674(02)00975-3. [DOI] [PubMed] [Google Scholar]
- 28.Muller J, Hart CM, Francis NJ, Vargas ML, Sengupta A, Wild B, Miller EL, O'Connor MB, Kingston RE, Simon JA. Cell. 2002;111:197–208. doi: 10.1016/s0092-8674(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds TY, Rockwell S, Glazer PM. Cancer Res. 1996;56:5754–5757. [PubMed] [Google Scholar]
- 30.Gibson SL, Bindra RS, Glazer PM. Cancer Res. 2005;65:10734–10741. doi: 10.1158/0008-5472.CAN-05-1160. [DOI] [PubMed] [Google Scholar]
- 31.Scher CD, Siegler R. Nature. 1975;253:729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- 32.Perucho M, Goldfarb M, Shimizu K, Lama C, Fogh J, Wigler M. Cell. 1981;27:467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- 33.Land H, Parada LF, Weinberg RA. Nature. 1983;304:596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- 34.Shalloway D, Johnson PJ, Freed EO, Coulter D, Flood WA., Jr Mol Cell Biol. 1987;7:3582–3590. doi: 10.1128/mcb.7.10.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cleary ML, Smith SD, Sklar J. Cell. 1986;47:19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- 36.Dyson PJ, Littlewood TD, Forster A, Rabbitts TH. EMBO J. 1985;4:2885–2891. doi: 10.1002/j.1460-2075.1985.tb04018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eick D, Bornkamm GW. EMBO J. 1989;8:1965–1972. doi: 10.1002/j.1460-2075.1989.tb03602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asimakopoulos FA, Shteper PJ, Krichevsky S, Fibach E, Polliack A, Rachmilewitz E, Ben-Neriah Y, Ben-Yehuda D. Blood. 1999;94:2452–2460. [PubMed] [Google Scholar]
- 39.Chess A, Simon I, Cedar H, Axel R. Cell. 1994;78:823–834. doi: 10.1016/s0092-8674(94)90562-2. [DOI] [PubMed] [Google Scholar]
- 40.Hollander GA, Zuklys S, Morel C, Mizoguchi E, Mobisson K, Simpson S, Terhorst C, Wishart W, Golan DE, Bhan AK, et al. Science. 1998;279:2118–2121. doi: 10.1126/science.279.5359.2118. [DOI] [PubMed] [Google Scholar]
- 41.Serizawa S, Ishii T, Nakatani H, Tsuboi A, Nagawa F, Asano M, Sudo K, Sakagami J, Sakano H, Ijiri T, et al. Nat Neurosci. 2000;3:687–693. doi: 10.1038/76641. [DOI] [PubMed] [Google Scholar]
- 42.Weng AP, Millholland JM, Yashiro-Ohtani Y, Arcangeli ML, Lau A, Wai C, Del Bianco C, Rodriguez CG, Sai H, Tobias J, et al. Genes Dev. 2006;20:2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.