Abstract
Recent studies that combined psychophysical/neurophysiological experiments [de Lafuente V, Romo R (2005) Nat Neurosci 8:1698–1703] analyzed the responses from single neurons, recorded in several cortical areas of parietal and frontal lobes, while trained monkeys reported the presence or absence of a mechanical vibration of varying amplitude applied to skin of one fingertip. The analysis showed that the activity of primary somatosensory cortex neurons covaried with the stimulus strength but did not covary with the animal's perceptual reports. In contrast, the activity of medial premotor cortex (MPC) neurons did not covary with the stimulus strength but did covary with the animal's perceptual reports. Here, we address the question of how perceptual detection is computed in MPC. In particular, we regard perceptual detection as a bistable neurodynamical phenomenon reflected in the activity of MPC. We show that the activity of MPC is consistent with a decision-making-like scenario of fluctuation-driven computation that causes a probabilistic transition between two bistable states, one corresponding to the case in which the monkey detects the sensory input, the other corresponding to the case in which the monkey does not. Moreover, the high variability activity of MPC neurons both within and between trials reflects stochastic fluctuations that may play a crucial role in the monkey's probabilistic perceptual reports.
Keywords: neurodynamics, perceptual detection, attractor networks, spiking dynamics, multistability
Since the foundation of experimental psychology, one of the core problems that marked the research of the mind was the question of how the brain translates sensory stimuli into meaningful interpretations. One refers to these brain processes as sensation and perception. Gustav Fechner (1) started to investigate quantitatively these brain processes establishing the functional relationship between the physical and the psychical world. A simpler form of operationalizing this problem is by means of the perceptual detection paradigm, i.e., the report of a produced or not produced sensory percept when a near-threshold sensory stimulus is presented. Recently, several studies reported the neural correlates of sensory detection by fMRI and single-cell recordings. In the case of vibrotactile stimulation, activity in somatosensory and frontal cortical areas suggests that perceptual detection results from different functional mechanisms, involving early sensory brain areas and cognitive processes (2, 3). In particular, de Lafuente and Romo (2) analyzed the recorded responses of neurons in primary somatosensory (S1) and medial premotor cortex (MPC) of monkeys judging the presence or absence of threshold vibrotactile stimulation. They observed that S1 neurons are always activated by the presence of the stimulus, but MPC neuronal activation covaries with the monkey's behavior. This observation suggests that S1 neurons encode the physical stimulation, whereas MPC neurons are engaged with the production of a sensory percept. Moreover, because neurons from MPC have been shown to be involved in the decision-making process during vibrotactile somatosensory discrimination (4), perceptual detection could be associated with a cognitive function, namely decision-making. Given these experimental facts, we can characterize the behavior associated with the phenomenon of perceptual detection and the underlying neural correlates. But, what is still missing are the computational mechanisms associated with those neural correlates and an answer to the question of how perceptual detection is computed.
The aim of this article is to analyze the putative computational mechanisms involved in perceptual detection. We focus our analysis on the paradigm and experimental results mentioned above (2). We analyze and model the activity of MPC neurons that correlates with perceptual detection by means of the theoretical framework proposed for decision making. In particular, we propose perceptual detection as the result of a neurodynamical bistability (5). Bistability refers to the regime in which two different stable states coexist. We show that the neural correlates underlying the production of a percept are consistent with a decision-making-like scenario of fluctuation-driven computation that causes probabilistic transition between two states: the “detection” and “no detection” of the sensory stimulus. We propose two possible bistable models. Moreover, one of the models predicts the existence of “no” neurons. We show experimental results that corroborate this prediction.
Results
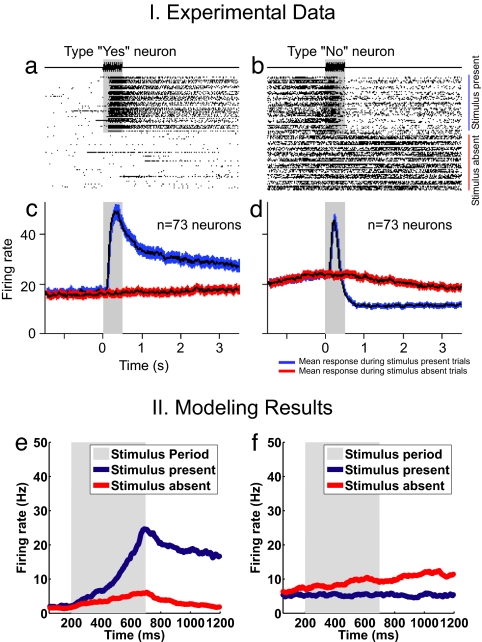
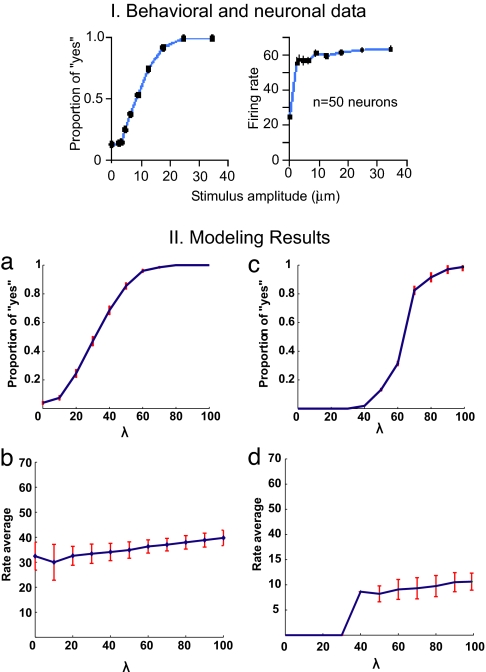
We present the results of the nonstationary probabilistic analysis calculated by means of the full spiking simulations averaged over several trials. In all cases, we aim to model the behavior of the MPC neurons that are shown in figure 3 c and f of the work of de Lafuente and Romo (2), which reflect the proper detection of the percept. We propose that the perceptual response results from a neurodynamical bistability. In this framework, each of the stable states corresponds to one possible perceptual response: “stimulus detected” or “stimulus not detected.” The probability of detecting the stimulus is given by the transitions between these two states. In fact, the probabilistic character of the system results from the stochastic nature of the networks. The finite-size effect is the source of this stochasticity. To compare the theoretical results with the experimental results, we first studied the characteristics of the bistable neurodynamical models. We plotted the behavior of the relevant populations encoding the different bistable states corresponding to the two alternative choices. For the NCYN model, Fig. 1IIa plots the proportion of “yes” responses as a function of the intensity of the applied vibrotactile stimulation, i.e., as a function of the strength λ of the stimulus presented. A similar result is obtained for the CYNN model (Fig. 1IIc). The two figures show that the proportion of “yes” responses (hits) increases as the intensity of the stimulus applied grows. Both models are consistent with the experimental results of de Lafuente and Romo (2) shown in their figure 3 e and f (reproduced here in Fig. 1I). Hence, both models show a probabilistic behavior that emulates the real behavior of subjects detecting a vibrotactile stimulus (2). Nevertheless, the CYNN shows a step function that is more consistent with the discharge characteristic of MPC neurons than the NCYN. Let us now concentrate on the level of firing activity observed in MPC neurons that covary with the behavioral responses. Fig. 1IIb for the NCYN model and Fig. 1IId for the CYNN model show the activity of the neurons encoding the “yes” response (selective excitatory population sensitive to the applied vibrotactile stimulus) averaged over trials that reported a percept (hits). In both models, the mean firing activity is almost constant and is not linearly related with the stimulus amplitude. On the contrary, the firing activity of the S1 neurons depends strongly on the stimulus amplitude. It is also reflected in the experimental results. The fact that neurons encoding the “yes” response present a relatively constant level of activation in trials that report a detected percept, whereas in trials that fail to detect a percept these neurons are low-activated (spontaneous level) is consistent with an attractor network. Therefore, the transition driven by the fluctuations are consistent with the behavioral data.
Fig. 3.
Responses of type “yes” and type “no” neurons recorded during stimulus-present (red line) and stimulus-absent (blue line) trials. (a) Raster plot of a “yes” neuron showing the strong responses to the vibratory stimulus and its maintained activity throughout the delay period preceding the behavioral response. (b) Raster plot of a “no” neuron showing the suppressed activity during the delay period and its maintained high firing rate during stimulus-absent trials. (c) Mean firing rate of 73 “yes” neurons during stimulus-present and stimulus-absent trials. (d) Mean firing rate of 71 “no” neurons during stimulus-present and stimulus-absent trials (black line indicates mean activity, and colored area depicts 95% confidence intervals). (e) Average activity over the “yes”-response trials of the population of neurons in the CYNN model that codifies the “yes” response. (f) Average activity over the “yes”-response trials of the population of neurons in the CYNN model that codifies the “no” response.
Fig. 1.
Experimental and simulated neural and behavioral data. I. Behavioral and neuronal data. Behavioral detection curve and responses to the stimulus of MPC neurons. (Left) Proportion of “yes” responses as a function of the amplitude of a 20-Hz vibratory stimulus applied to a fingertip (500 trials for each noncero amplitude and 4,500 trials for the cero amplitude). (Right) Mean response of MPC neurons as a function of stimulus amplitude. In contrast to what has been observed in primary somatosensory areas, stimulus amplitude has little effect on the activity of MPC neurons. II. Modeling results. NCYN model (a and b) and CYNN model (c and d).
Model Predictions and Experimental Recordings: “Yes” Neurons and “No” Neurons.
Despite the fact that both models are consistent with the existing experimental data (2), the underlying computation is totally different. In one case, the detection of a percept is associated with the probability of activation of a bistable population in the absence of strong competitions (model NCYN). On the other hand, the underlying computation in the CYNN corresponds to a more genuine decision-making paradigm because the bistability is given by a strong competition with the extra excitatory population encoding a default “no” response. Both models can be distinguished, because they perform different predictions when the detailed underlying temporal evolution of the whole network is investigated.
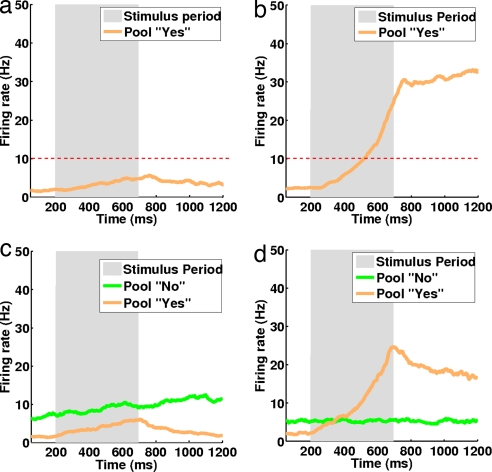
A typical misstrial corresponding to a “no” response (perception not detected) is depicted in Fig. 2a. In this case, the specific excitatory population sensitive to the applied vibrotactile stimulus remains in the spontaneous state. Fig. 2b shows a typical hit trial, i.e., a “yes” response (perception detected). The specific excitatory population sensitive to the applied vibrotactile stimulus reflects a transition to a high firing-rate state encoding the “yes” response. In both cases, the stimulus is applied between 200 and 700 ms (shadowed region). The red dashed line marks the value of the threshold we used to classify the response.
Fig. 2.
Temporal evolution of the firing-rate activity of the selective excitatory population sensitive to the specific vibrotactile stimulation applied for two different trials with NCYN (Upper) and CYNN (Lower) models. (Left) “No” response (perception not detected). Pearson's correlation computed for the two pools during a “no” response trial is 0.63 for model CYNN. (Right) “Yes” response (perception detected) and a Pearson's correlation of 0.66 for CYNN. Stimulus applied between 200 and 700 ms (shadowed region). Red dashed line on the NCYN marks the value of the classification threshold. The simulations have been performed by the full spiking and synaptic simulation of the network. The orange curves correspond to the “yes” pool and green curves correspond to the “no” pool.
Fig. 2 c and d show similar simulations, but now for the CYNN model. In this case, we plotted the temporal evolution of the firing-rate activity of the selective excitatory population sensitive to the specific vibrotactile stimulation applied and the extra excitatory population encoding the default “no” response. Fig. 2c plots a typical miss trial corresponding to a “no” response (perception not detected). There is no transition, and the “no”-response neurons increase their firing activity monotonically and remain strongly activated (encoding a no-detection response). Their activity will tend to decrease again after a period. In the case of the “yes”-response neurons, their firing activity is inhibited, and remains at a low level. Fig. 2d shows a typical hit trial, i.e., a “yes” response (perception detected). As before, the specific “yes” neurons, sensitive to the applied vibrotactile stimulus, perform a transition to a high activity state, whereas the “no” neurons are slightly inhibited, and they fire at an activity lower than the one they show in the prestimulus period. This state encodes the “yes” response. In this model, the “no” response is reflected by an active population that shows an elevated level of firing activity during the pre- and poststimulation period, whereas the “yes” response population goes to a state of low firing-rate activity. In other words, in addition to the model NCYN, which just reflects the neurodynamics of the “yes” neurons, the CYNN model can capture the dynamics of the “no” neurons, which is a concrete prediction that can be identified experimentally.
In addition to the “yes”-response neurons, and in agreement with the CYNN, we found that half of the recorded MPC neurons (71 of 144, 49%) suppressed their activity during the delay period after stimulus presentation (figure 2a MPC, middle column in ref. 6). These neurons showed high basal activity that was maintained if no stimulus was presented (Fig. 3I). The firing dynamics of these neurons support the idea that decisions about stimulus presence or absence are coded by two distinct neuronal populations engaged in a competition that determines the final behavioral output, as postulated by the CYNN model. Notice that in the experimental plots, MPC “no” neurons show a peak of activity when a stimulus is applied that is not captured by the CYNN model. We hypothesize that this sudden increase of activity is due to the fact that, during the stimulation period all neurons (“yes”/“no” neurons) show strong activation encoding the presence of the stimulus. Nevertheless, after this period of stimulation, the neuronal system engages in a decision-making process that is the one that we are modeling here. Therefore, the different type of firing-activity modulations observed in the “yes” neurons and “no” neurons after the stimulation is perfectly captured by the CYNN model. The fact that “no”-response neurons showed increased basal firing rates is consistent with the idea that the decision to answer “no” is a default decision which competes against the decision to answer “yes.” These experimental observations strongly support the computation of the detection consistent with model CYNN.
Discussion
In this article, we show that perceptual detection results from a decision-making-like cognitive operation associated with a multistable neurodynamical phenomenon. The neural correlates underlying the production of a percept are consistent with a scenario of fluctuation-driven computation that causes probabilistic transitions between multistable states, corresponding to detection or no detection of the sensory stimulus. Our model begins to describe the mechanisms underlying the link between the neuronal stochasticity and the behavior by constructing computational models that account for both cellular and behavioral levels. In this way, we extend computational and theoretical neuroscience approaches to the problem of decision making. These approaches used biophysically realistic neural circuits designed to implement stochastic noise-driven decision-making (7–9). In general, these models involve populations of excitatory neurons engaged in competitive interactions mediated by inhibition. The external physical stimuli bias the competition in favor of one of the populations that develops gradually increased activity whereas activity in the other populations is inhibited. This final configuration corresponds to a decision state associated with a specific choice. In this scenario, the strong and weak activity states are stable for the same set of parameter values, which is called bistability. The computation involved in decision making is then understood as the fluctuation-driven, probabilistic transition from the spontaneous to the decision state. These biophysical neurodynamical implementations are able to qualitatively account for some experimental aspects of psychometric and neurometric data underlying decision making (10).
In particular, we proposed two different network models: noncompeting “yes” neurons (NCYN) and competing “yes–no” neurons (CYNN). Both models are consistent with the existing single-cell recordings, but they involve different types of bistable decision states and, consequently, different types of computation and neurodynamics. By analyzing the temporal evolution of the firing-rate activity of neurons in trials associated with the two different behavioral responses, we were able to show experimental evidences in favor of the CYNN model. Specifically, the CYNN model predicts the existence of neurons that encode the “no” response and others associated with the “yes” response. The first ones slightly decrease their activity at the end of the trial, whereas the second group increases their firing activity when a stimulus is presented. In conclusion, the computational models proposed here provide a deeper understanding of the fundamental mechanisms underlying perceptual detection and how these are related with the neuroscience data. We argue that addressing such a task is a prerequisite for grounding empirical neuroscience in a cogent theoretical framework.
Materials and Methods
Neuronal Correlates of Perceptual Detection.
We focus on the experimental results of the neural correlates (S1 and MPC areas) of subjective sensory experience (2). de Lafuente and Romo used a behavioral task where trained awake monkeys report the presence or absence of a mechanical vibration applied to their fingertips by pressing one of two pushbuttons. de Lafuente and Romo found that the activity of MPC neurons was only weakly modulated by the stimulus amplitude, and it covaried with the monkeys' trial-by-trial reports. On the contrary, S1 neurons did not covary with the animals' perceptual reports, but their firing rate did show a monotonically increasing graded dependence with the stimulus amplitude (figure 3 c and f in ref. 2). The fact that MPC neurons correlate with the behavioral performance, but show an all-or-none firing-rate response, suggests an underlying bistable dynamic in an attractor framework.
Stochastics Neurodynamical Model.
The neurodynamical framework we use here consists of a network of integrate-and-fire neurons with realistic synaptic dynamics. In the model, competition and cooperation mechanisms can account for the most-relevant characteristics of the neuronal activity related with subjective sensory experience. These competition and cooperation mechanisms are implemented in an attractor network consisting of recurrently connected populations of excitatory neurons mutually connected with a common inhibitory population. This neurodynamical formulation is based on the principle of biased competition/cooperation, which has been able to simulate and explain, in a unifying framework, attention, working memory, reward processing in a variety of tasks (5, 11, 15), and decision-making (7, 15).
Network.
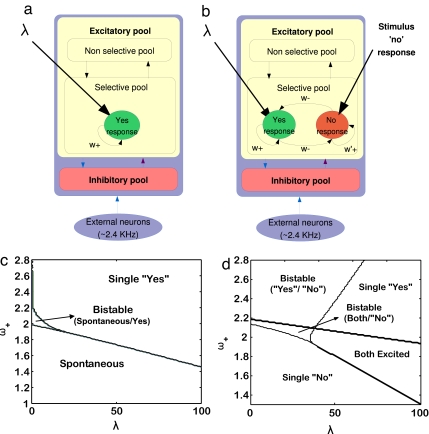
We modeled a patch of MPC neurons in the frontal lobe by a network of interacting neurons organized into a discrete set of populations. Populations are defined as groups of excitatory or inhibitory neurons sharing the same inputs and connectivities. Some of the excitatory population of neurons have a selective response that, in our case, reflects the sensitivity to an external applied vibrotactile stimulation. All other excitatory neurons are grouped in a “nonselective” population. There is also one inhibitory population grouping the local inhibitory neurons that regulate the overall activity by implementing competition in the network. Neurons in the networks are connected by three types of receptors that mediate the synaptic currents flowing into them: AMPA, NMDA glutamate, and GABA receptors. Neurons within a specific excitatory population are mutually coupled with a strong weight ω+. Neurons between two different selective populations have anticorrelated activity that results in weaker connections ω−.
We propose two different network models that are consistent with the two possible behavioral responses: “stimulus detection” and “no stimulus detection,” The computation involved in perceptual detection is then understood as the fluctuation-driven, probabilistic transition to one of the two possible bistable decision states. In the first model, a selective excitatory population corresponds to the detection of a percept associated with an external applied vibrotactile stimulation. We assume that the strength of the input impinging in that excitatory population λ is proportional to the strength of the presented vibrotactile stimulus (as, for example, encoded in S1, i.e., the input to MPC is transmitted from S1). When a stimulus is presented, there is just one population sensitive to it. To model this characteristic, we use a network composed of two selective populations, but only one will be selective to the stimuli applied (in fact, in this first model, the neurons in the second selective populations behave as the nonselective neurons). The relevant bistability in this model is therefore given by the state where the excitatory populations are low activated (corresponding to no detection of a percept, i.e., “no” response) and the state where the excitatory population sensitive to the presented vibrotactile stimulus is highly activated (corresponding to the detection of the percept, i.e., “yes” response). We denominate this model noncompeting “yes” neurons (NCYN) (Fig. 4a). Just the selective population sensitive to the applied vibrotactile stimulation used in the experiment is represented. In the second model, we add to the network extra excitatory populations corresponding to a “no detection” default response. We denominate this model competing “yes–no” neurons (CYNN) (Fig. 4b). The figure shows a selective excitatory population sensitive to the applied vibrotactile stimulation used in the experiment (corresponding to the detection of the percept, i.e., “yes” response) and the extra selective excitatory population encoding the “no” response (corresponding to the not detection of a percept, i.e., “no” response). The latter pool receives a constant input of 50 Hz (“no detection” default input) encoding the “no” response by default. This input causes a competition between the neurons sensitive to an external applied vibrotactile stimulation and the neurons that encodes the default “no” response. In this case, the dynamics corresponds to a genuine decision-making competitive computation between the neurons sensitive to an external applied vibrotactile stimulation and the neurons that encode the “no” response by default. The relevant bistability is therefore given by one state where the excitatory population corresponding to the “no” response is highly activated, and the excitatory population sensitive to the presence of the specific stimulus is inhibited (“no stimulus detection”). Another state appears when the “no” population is inhibited and the specific population sensitive to the stimulus wins the competition (“stimulus detection”). Note that in the CYNN model, we use different cohesion values ω+ and ω′+ for the different selective populations and the same ω−. See the supporting information (SI) for a full specification of the whole connectivity.
Fig. 4.
Network architecture and corresponding bifurcation diagrams. (Upper) Minimal network architecture of the MPC model. The two proposed models are shown in a and b. (a) The model NCYN has excitatory populations selective to the applied vibrotactile stimulation. A “no” response is given when the selective population is low activated and a “yes” response is given when it is high activated. (b) The CYNN model has excitatory selective populations encoding the “yes” response selective to the stimulus applied and a selective population encoding a “no” response by default. The “no” by default is modeled as a constant bias to the population that codifies the “no” response. A “yes” response is given when the population sensitive to the frequency applied is high activated over the population that codifies the “no” response. A “no” response is given when the population sensitive to the stimulus is inhibited by the population that codifies the “no” response. The arrows indicate the recurrent connections between the different neurons in a pool. (Lower) Bifurcation diagrams for both NYCN (c) and CYNN (d) models. The diagrams show the different attractor regions as a function of the stimulus input (λ) and the level of coupling within the neurons of the same selective population (cohesion).
Simulations.
We study the characteristics of the network in the stationary conditions with the mean-field approach. Using this approximation, we scan the relevant parameter space given by the population cohesion ω+ versus the external input λ. The mean-field results for the NCYN-model are illustrated in a bifurcation diagram (Fig. 4c) that shows different regimes of the network. For small values of λ and for a weak population cohesion, the network has one stable state where all populations are firing at a weak level (spontaneous state). This spontaneous state encodes the “no” response in the NYCN model. For higher population cohesion and higher values of λ, a state corresponding to the strong activation of the selective population sensitive to the applied vibrotactile stimulation emerges. We call this excited state encoding the “yes” response the “yes” state. Between these two regions, there is a bistable region where the state corresponding to weak (“no” response) or strong (“yes” response) activation states of the selective population sensitive to the applied vibrotactile stimulation are both stable. For the CYNN model, the mean-field analysis is summarized in Fig. 4d. In this case, the bifurcation diagram has five different regions: three unique stable-state regions and two bistable regions. We distinguish a unique stable “yes”-state region (“yes”-response population strongly activated/“no”-response population weakly activated), another one with a “no”-state region (“no”-response population strongly activated/“yes”-response population weakly activated) and a region in which both populations are excited (“yes”-response population strongly activated/“no”-response population strongly activated, a unique stable state, too). In the first bistable region, the “yes” state and the “no” state coexist, and in the other one, the coexistence is between the “no” state and a state in which both populations are excited. Once we analyzed the bifurcation diagrams, we restricted the range of parameters selecting those in which the networks operate in the bistable regime.
To study the probabilistic behavior of the neuronal dynamics of the network, we analyzed the spiking simulations of the configurations corresponding to the region of bistability. We analyzed the spiking data consistently with the analysis of the experiment data in ref. 2 (see the SI).
Supplementary Material
Acknowledgments
R.R.'s research was partially supported by an International Research Scholars Award from the Howard Hughes Medical Institute and grants from the Dirección del Personal Académico de la Universidad Nacional Autónama de México and the Consejo National de Ciencia y Tecnología. On Tuesday, November 16, 2006, G.D.'s research was supported by the European project “Decision in Motion.”
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709794104/DC1.
References
- 1.Fechner G. Elements der Psychophysik. Leipzig, Germany: Breitkof un Härtel; 1860. [Google Scholar]
- 2.de Lafuente V, Romo R. Nat Neurosci. 2005;8:1698–1703. doi: 10.1038/nn1587. [DOI] [PubMed] [Google Scholar]
- 3.Shulman G, Ollinger J, Linenweber M, Petersen S, Corbetta M. Proc Natl Acad Sci USA. 2001;98:313–318. doi: 10.1073/pnas.021381198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez A, Zainos A, Romo R. Neuron. 2002;33:959–972. doi: 10.1016/s0896-6273(02)00613-x. [DOI] [PubMed] [Google Scholar]
- 5.Deco G, Rolls E. J Neurophysiol. 2005;94:295–313. doi: 10.1152/jn.01095.2004. [DOI] [PubMed] [Google Scholar]
- 6.de Lafuente V, Romo R. Proc Natl Acad Sci USA. 2006;103:144266–144271. doi: 10.1073/pnas.0605826103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang XJ. Neuron. 2002;36:955–968. doi: 10.1016/s0896-6273(02)01092-9. [DOI] [PubMed] [Google Scholar]
- 8.Brody C, Romo R, Kepecs A. Curr Opin Neurobiol. 2003;13:204–211. doi: 10.1016/s0959-4388(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 9.Machens C, Romo R, Brody C. Science. 2005;307:1121–1124. doi: 10.1126/science.1104171. [DOI] [PubMed] [Google Scholar]
- 10.Romo R, Salinas E. Nat Rev Neurosci. 2003;4:203–218. doi: 10.1038/nrn1058. [DOI] [PubMed] [Google Scholar]
- 11.Rolls ET, Deco G. Computational Neuroscience of Vision. Oxford: Oxford Univ Press; 2002. [Google Scholar]
- 12.Deco G, Rolls E. Eur J Neurosci. 2003;18:2374–2390. doi: 10.1046/j.1460-9568.2003.02956.x. [DOI] [PubMed] [Google Scholar]
- 13.Deco G, Rolls E. Vision Res. 2004;44:621–644. doi: 10.1016/j.visres.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 14.Deco G, Rolls ET, Horwitz B. J Cognit Neurosci. 2004;16:683–701. doi: 10.1162/089892904323057380. [DOI] [PubMed] [Google Scholar]
- 15.Wong K, Wang X. J Neurosci. 2006;26:1314–1328. doi: 10.1523/JNEUROSCI.3733-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.