Abstract
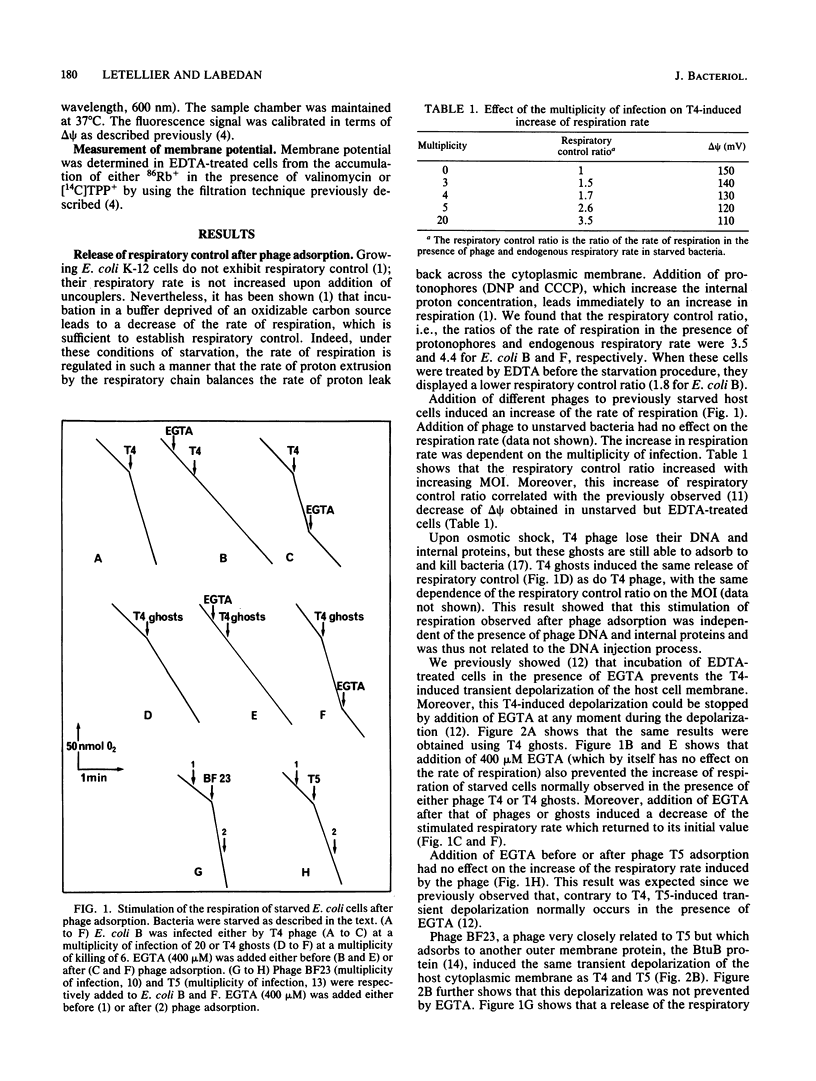
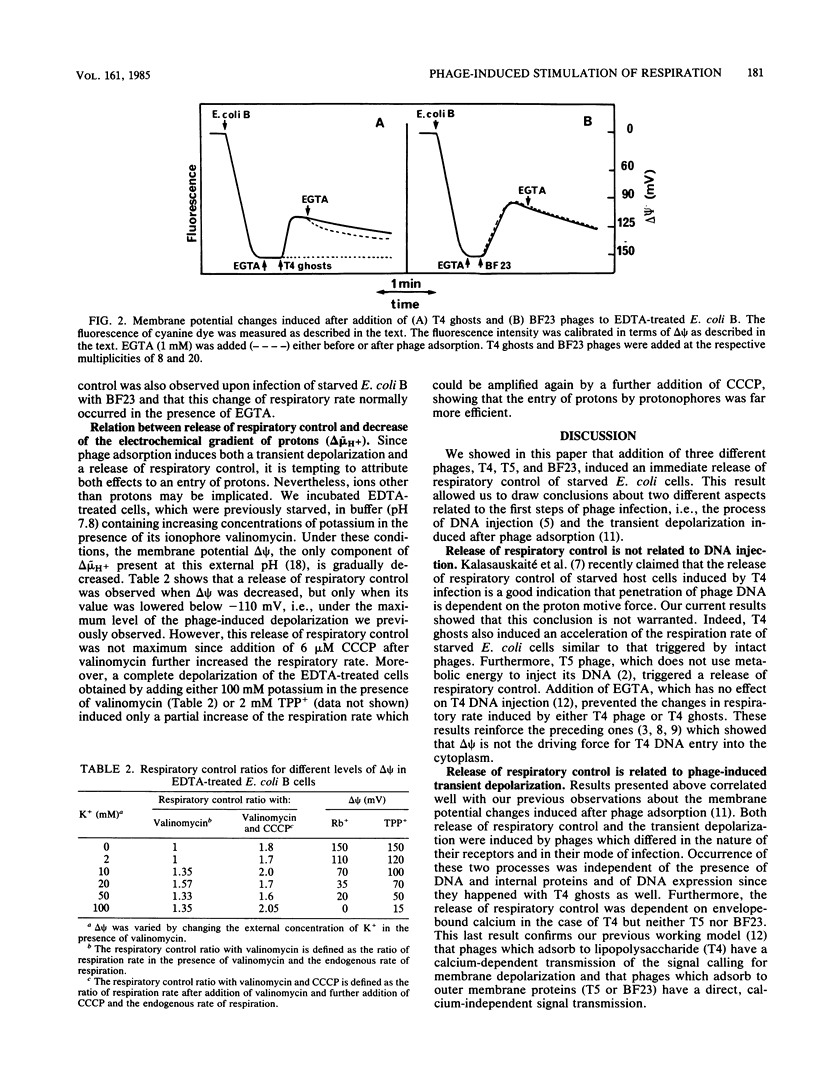
Adsorption of phages T4, T5, and BF23 to previously starved Escherichia coli cells triggered the immediate release of respiratory control. A similar stimulation of respiration was induced after T4 ghost attachment, showing that this process was independent of the mechanism of DNA injection. Rather, this change in the respiratory rate was related to the transient depolarization of the cytoplasmic membrane also induced after phage and ghost adsorption. Both processes were suppressed by addition of ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid in the case of T4 (phage and ghosts) but not of T5 and BF23. The increase in respiratory rate observed after phage adsorption was of a magnitude similar to that induced by protonophores. Since other treatments that depolarize the membrane without a massive proton influx did not increase the rate of respiration of starved bacteria with the same efficiency, these results suggest that phage adsorption induced an entry of protons into the cell cytoplasm.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burstein C., Tiankova L., Kepes A. Respiratory control in Escherichia coli K 12. Eur J Biochem. 1979 Mar;94(2):387–392. doi: 10.1111/j.1432-1033.1979.tb12905.x. [DOI] [PubMed] [Google Scholar]
- Filali Maltouf A., Labedan B. Host cell metabolic energy is not required for injection of bacteriophage T5 DNA. J Bacteriol. 1983 Jan;153(1):124–133. doi: 10.1128/jb.153.1.124-133.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H., Kuroiwa T., Mizushima S. DNA injection during bacteriophage T4 infection of Escherichia coli. J Bacteriol. 1983 May;154(2):938–945. doi: 10.1128/jb.154.2.938-945.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazi A., Schechter E., Letellier L., Labedan B. Probes of membrane potential in Escherichia coli cells. FEBS Lett. 1981 Mar 23;125(2):197–200. doi: 10.1016/0014-5793(81)80717-x. [DOI] [PubMed] [Google Scholar]
- Kalasauskaite E. V., Kadisaite D. L., Daugelavicius R. J., Grinius L. L., Jasaitis A. A. Studies on energy supply for genetic processes. Requirement for membrane potential in Escherichia coli infection by phage T4. Eur J Biochem. 1983 Jan 17;130(1):123–130. [PubMed] [Google Scholar]
- Kalasauskaite E., Grinius L. The role of energy-yielding ATPase and respiratory chain at early stages of bacteriophage T4 infection. FEBS Lett. 1979 Mar 15;99(2):287–291. doi: 10.1016/0014-5793(79)80974-6. [DOI] [PubMed] [Google Scholar]
- Labedan B., Goldberg E. B. Requirement for membrane potential in injection of phage T4 DNA. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4669–4673. doi: 10.1073/pnas.76.9.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labedan B., Heller K. B., Jasaitis A. A., Wilson T. H., Goldberg E. B. A membrane potential threshold for phage T4 DNA injection. Biochem Biophys Res Commun. 1980 Mar 28;93(2):625–630. doi: 10.1016/0006-291x(80)91124-9. [DOI] [PubMed] [Google Scholar]
- Labedan B., Legault-Demare J. Evidence for heterogeneity in populations of T5 bacteriophage. J Virol. 1974 May;13(5):1093–1100. doi: 10.1128/jvi.13.5.1093-1100.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labedan B., Letellier L. Membrane potential changes during the first steps of coliphage infection. Proc Natl Acad Sci U S A. 1981 Jan;78(1):215–219. doi: 10.1073/pnas.78.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letellier L., Labedan B. Involvement of envelope-bound calcium in the transient depolarization of the Escherichia coli cytoplasmic membrane induced by bacteriophage T4 and T5 adsorption. J Bacteriol. 1984 Mar;157(3):789–794. doi: 10.1128/jb.157.3.789-794.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letellier L., Shechter E. Cyanine dye as monitor of membrane potentials in Escherichia coli cells and membrane vesicles. Eur J Biochem. 1979 Dec 17;102(2):441–447. doi: 10.1111/j.1432-1033.1979.tb04259.x. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- O'Shea P. S., Chappell J. B. The relationship between the rate of respiration and the protonmotive force. The role of proton conductivity. Biochem J. 1984 Apr 15;219(2):401–404. doi: 10.1042/bj2190401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Duckworth D. H. Metabolism of T4 bacteriophage ghost-infected cells: effect of bacteriophage and ghosts on the uptake of carbohydrates in Escherichia coli B. J Bacteriol. 1971 Jul;107(1):259–267. doi: 10.1128/jb.107.1.259-267.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D., Schuldiner S., Padan E. Proton electrochemical gradient in Escherichia coli cells and its relation to active transport of lactose. Biochemistry. 1979 Feb 20;18(4):669–673. doi: 10.1021/bi00571a018. [DOI] [PubMed] [Google Scholar]


