Abstract
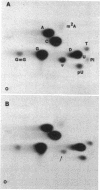
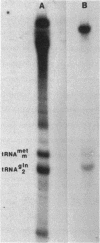
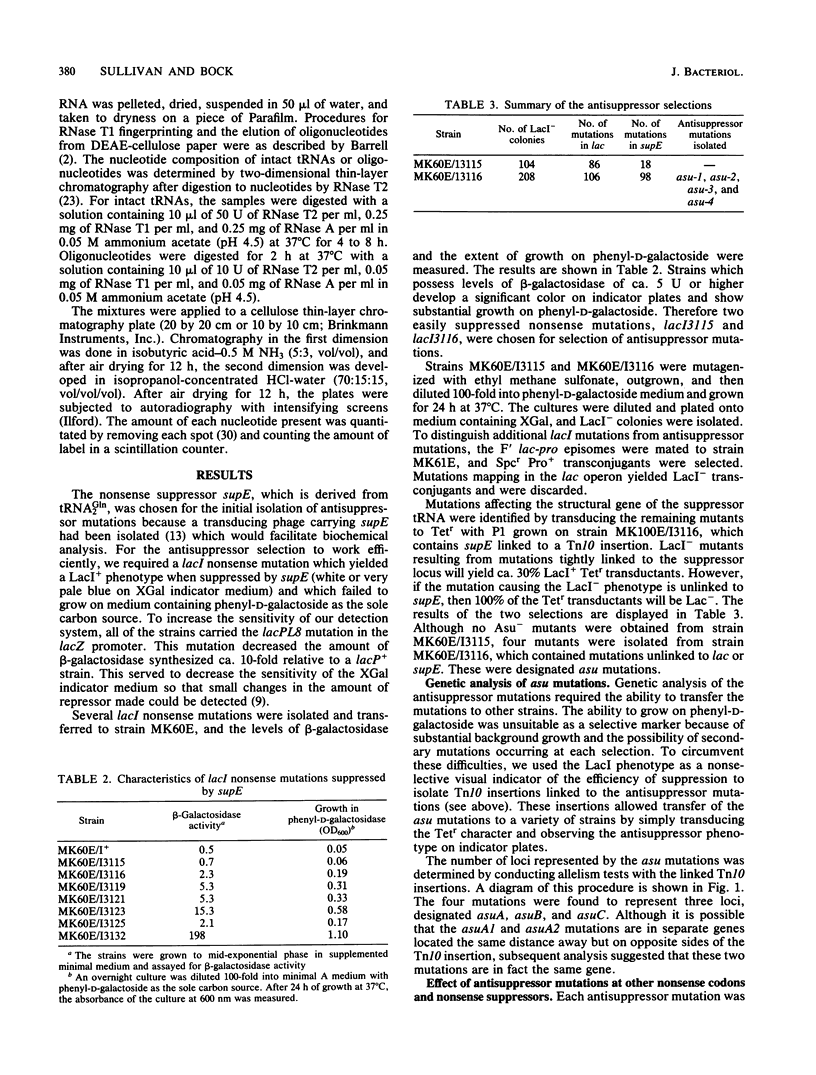
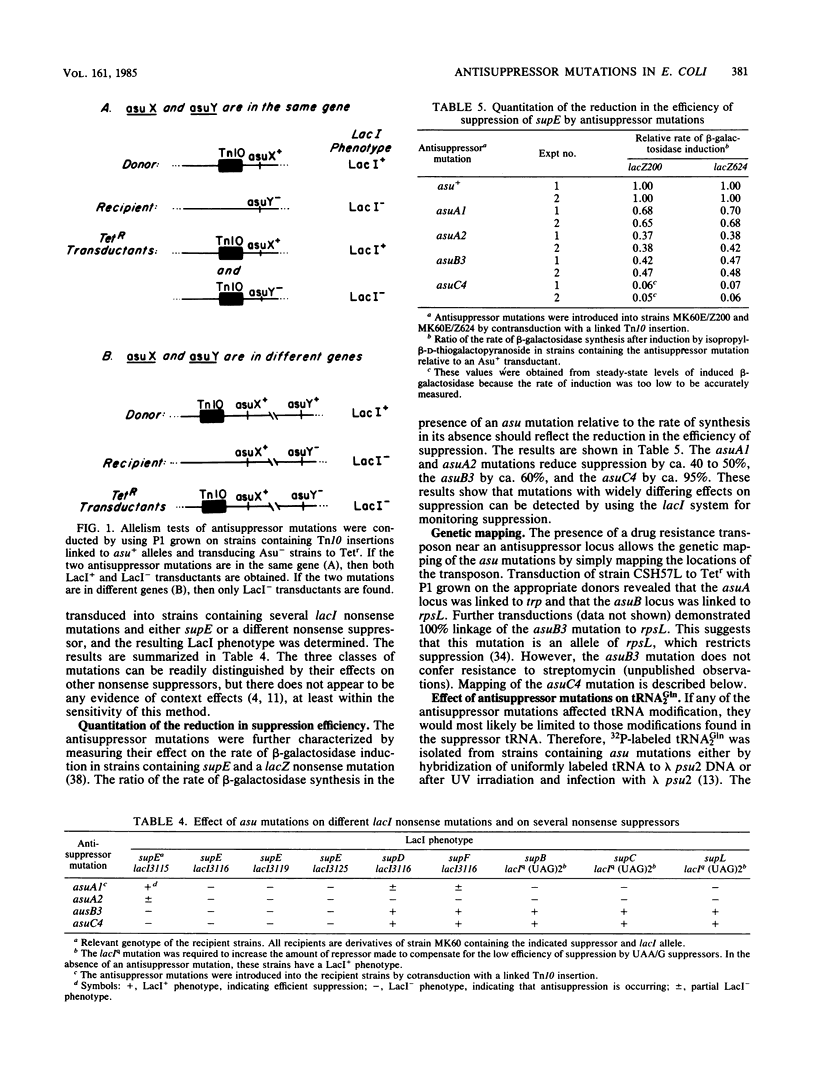
Nonsense mutations in lacI have been shown to be useful as indicators of the efficiency of nonsense suppression. From strains containing supE and a lacI nonsense mutation, selection for LacI- mutants has resulted in the isolation of four antisuppressor mutations. Tn10 insertions linked to these mutations were isolated and used to group the four mutations into three loci. The asuA1 and asuA2 mutations are linked to trp, reduce suppression by supE approximately twofold, and affect a variety of suppressors. The asuB3 mutation was mapped by P1 cotransduction to rpsL but does not confer resistance to streptomycin. The asuC4 mutation reduced suppression by supE by 95% and was shown biochemically to result in the loss of two pseudouridine modifications from the 3' side of the anticodon stem and loop of tRNA2Gln. This mutation is linked to purF, suggesting that it is a new allele of hisT.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi L., Ruth J. R. The influence of codon context on genetic code translation. Nature. 1980 Jul 10;286(5769):123–127. doi: 10.1038/286123a0. [DOI] [PubMed] [Google Scholar]
- Bradley D., Park J. V., Soll L. TRNA2Gln Su+2 mutants that increase amber suppression. J Bacteriol. 1981 Feb;145(2):704–712. doi: 10.1128/jb.145.2.704-712.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni C. B., Colantuoni V., Sbordone L., Cortese R., Blasi F. Biochemical and regulatory properties of Escherichia coli K-12 hisT mutants. J Bacteriol. 1977 Apr;130(1):4–10. doi: 10.1128/jb.130.1.4-10.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby D. S., Schedl P., Guthrie C. A functional requirement for modification of the wobble nucleotide in tha anticodon of a T4 suppressor tRNA. Cell. 1976 Nov;9(3):449–463. doi: 10.1016/0092-8674(76)90090-8. [DOI] [PubMed] [Google Scholar]
- Cortese R., Kammen H. O., Spengler S. J., Ames B. N. Biosynthesis of pseudouridine in transfer ribonucleic acid. J Biol Chem. 1974 Feb 25;249(4):1103–1108. [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. III. Additional correlation of mutational sites with specific amino acid residues. J Mol Biol. 1977 Dec 15;117(3):525–567. doi: 10.1016/0022-2836(77)90056-0. [DOI] [PubMed] [Google Scholar]
- Feinstein S. I., Altman S. Context effects on nonsense codon suppression in Escherichia coli. Genetics. 1978 Feb;88(2):201–219. doi: 10.1093/genetics/88.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss D. H., Sprinzl M. Compilation of tRNA sequences. Nucleic Acids Res. 1983 Jan 11;11(1):r1–53. [PMC free article] [PubMed] [Google Scholar]
- Inokuchi H., Yamao F., Sakano H., Ozeki H. Identification of transfer RNA suppressors in Escherichia coli. I. Amber suppressor su+2, an anticodon mutant of tRNA2Gln. J Mol Biol. 1979 Aug 25;132(4):649–662. doi: 10.1016/0022-2836(79)90380-2. [DOI] [PubMed] [Google Scholar]
- Johnston H. M., Barnes W. M., Chumley F. G., Bossi L., Roth J. R. Model for regulation of the histidine operon of Salmonella. Proc Natl Acad Sci U S A. 1980 Jan;77(1):508–512. doi: 10.1073/pnas.77.1.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Barker D. F., Ross D. G., Botstein D. Properties of the translocatable tetracycline-resistance element Tn10 in Escherichia coli and bacteriophage lambda. Genetics. 1978 Nov;90(3):427–461. doi: 10.1093/genetics/90.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Landy A., Abelson J., Goodman H. M., Smith J. D. Specific hybridization of tyrosine transfer ribonucleic acids with DNA from a transducing bacteriophage phi-80 carrying the amber suppressor gene su 3. J Mol Biol. 1967 Nov 14;29(3):457–471. doi: 10.1016/0022-2836(67)90112-x. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R., Söll D., Kwong T. C. Isolation and partial characterization of three Escherichia coli mutants with altered transfer ribonucleic acid methylases. J Bacteriol. 1975 Apr;122(1):257–265. doi: 10.1128/jb.122.1.257-265.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menninger J. R., Walker C., Tan P. F. Studies on the metabolic role of peptidyl-tRNA hydrolase. I. Properties of a mutant E. coli with temperature-sensitive peptidyl-tRNA hydrolase. Mol Gen Genet. 1973 Mar 19;121(4):307–324. doi: 10.1007/BF00433230. [DOI] [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Noguchi S., Yamaizumi Z., Ohgi T., Goto T., Nishimura Y., Hirota Y., Nishimura S. Isolation of Q nucleoside precursor present in tRNA of an E. coli mutant and its characterization as 7-(cyano)-7-deazaguanosine. Nucleic Acids Res. 1978 Nov;5(11):4215–4223. doi: 10.1093/nar/5.11.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D. T., Blum P. H., Artz S. W. Effects of the hisT mutation of Salmonella typhimurium on translation elongation rate. J Bacteriol. 1983 Jan;153(1):357–363. doi: 10.1128/jb.153.1.357-363.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Yamada S., Ikemura T., Shimura Y., Ozeki H. Temperature sensitive mutants of Escherichia coli for tRNA synthesis. Nucleic Acids Res. 1974 Mar;1(3):355–371. doi: 10.1093/nar/1.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl P., Primakoff P. Mutants of Escherichia coli thermosensitive for the synthesis of transfer RNA. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2091–2095. doi: 10.1073/pnas.70.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman J. G., Barrell B. G., McClain W. H. Five steps in the conversion of a large precursor RNA into bacteriophage proline and serine transfer RNAs. J Mol Biol. 1975 Dec 25;99(4):733–760. doi: 10.1016/s0022-2836(75)80182-3. [DOI] [PubMed] [Google Scholar]
- Smith J. D., Barnett L., Brenner S., Russell R. L. More mutant tyrosine transfer ribonucleic acids. J Mol Biol. 1970 Nov 28;54(1):1–14. doi: 10.1016/0022-2836(70)90442-0. [DOI] [PubMed] [Google Scholar]
- Smith T. F., Sadler J. R. The nature of lactose operator constitive mutations. J Mol Biol. 1971 Jul 28;59(2):273–305. doi: 10.1016/0022-2836(71)90051-9. [DOI] [PubMed] [Google Scholar]
- Sternglanz R., DiNardo S., Voelkel K. A., Nishimura Y., Hirota Y., Becherer K., Zumstein L., Wang J. C. Mutations in the gene coding for Escherichia coli DNA topoisomerase I affect transcription and transposition. Proc Natl Acad Sci U S A. 1981 May;78(5):2747–2751. doi: 10.1073/pnas.78.5.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigini P., Gorini L. Ribosomal mutations affecting efficiency of amber suppression. J Mol Biol. 1970 Feb 14;47(3):517–530. doi: 10.1016/0022-2836(70)90319-0. [DOI] [PubMed] [Google Scholar]
- Sullivan M. A., Cannon J. F., Webb F. H., Bock R. M. Antisuppressor mutation in Escherichia coli defective in biosynthesis of 5-methylaminomethyl-2-thiouridine. J Bacteriol. 1985 Jan;161(1):368–376. doi: 10.1128/jb.161.1.368-376.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Neill R. J., Landsberg R., Ames B. N. Pseudouridylation of tRNAs and its role in regulation in Salmonella typhimurium. J Biol Chem. 1979 Jun 25;254(12):5111–5119. [PubMed] [Google Scholar]
- Weiner A. M. An abundant cytoplasmic 7S RNA is complementary to the dominant interspersed middle repetitive DNA sequence family in the human genome. Cell. 1980 Nov;22(1 Pt 1):209–218. doi: 10.1016/0092-8674(80)90169-5. [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Lindahl L. High-efficiency, temperature-sensitive suppression of amber mutations in Escherichia coli. J Bacteriol. 1981 Jan;145(1):459–465. doi: 10.1128/jb.145.1.459-465.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]