Abstract
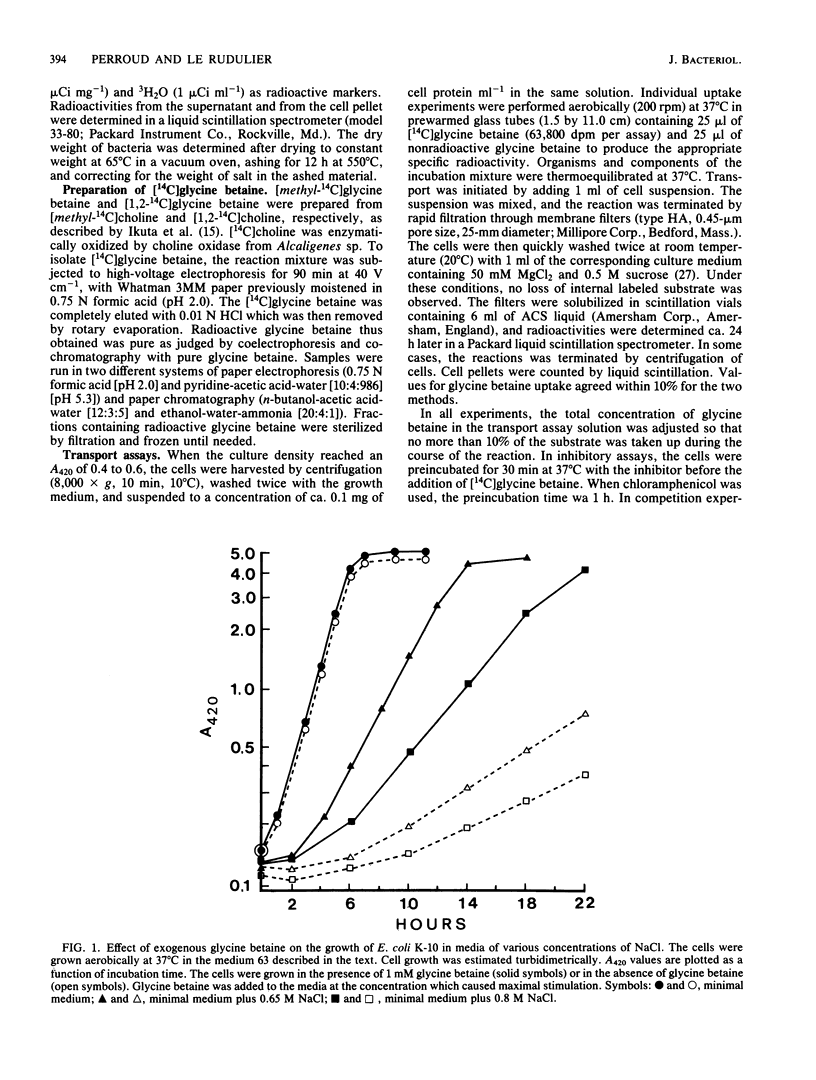
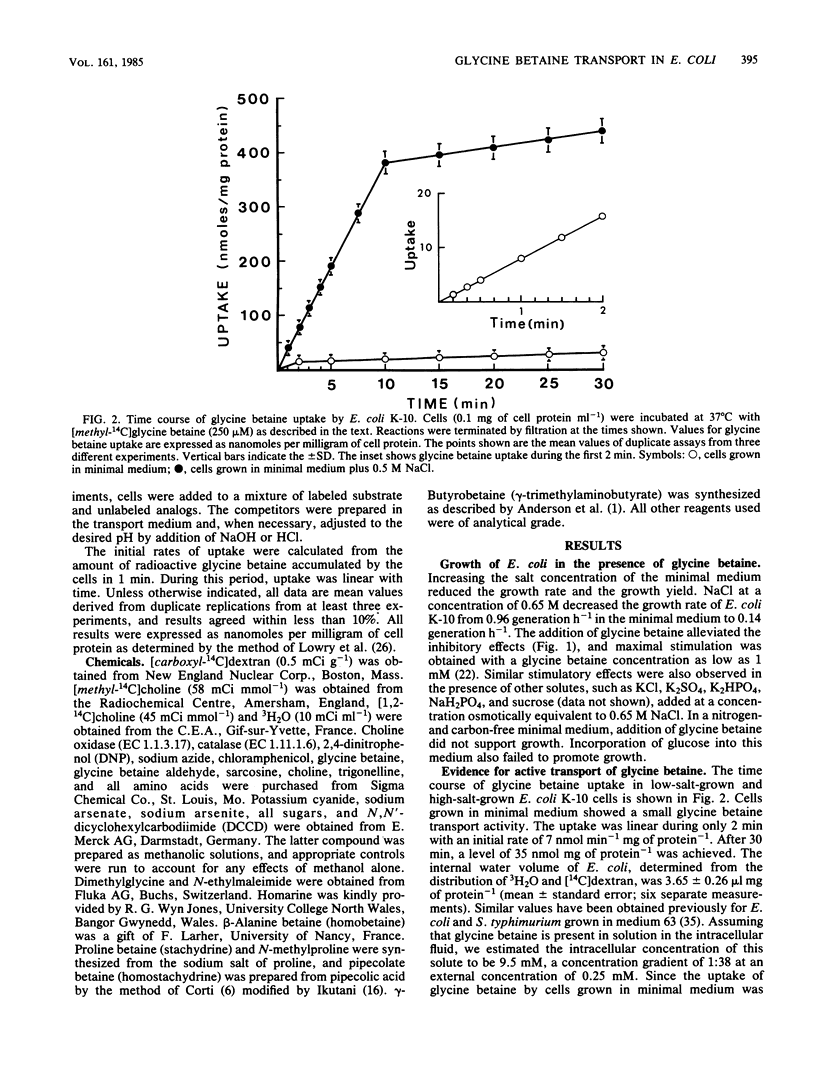
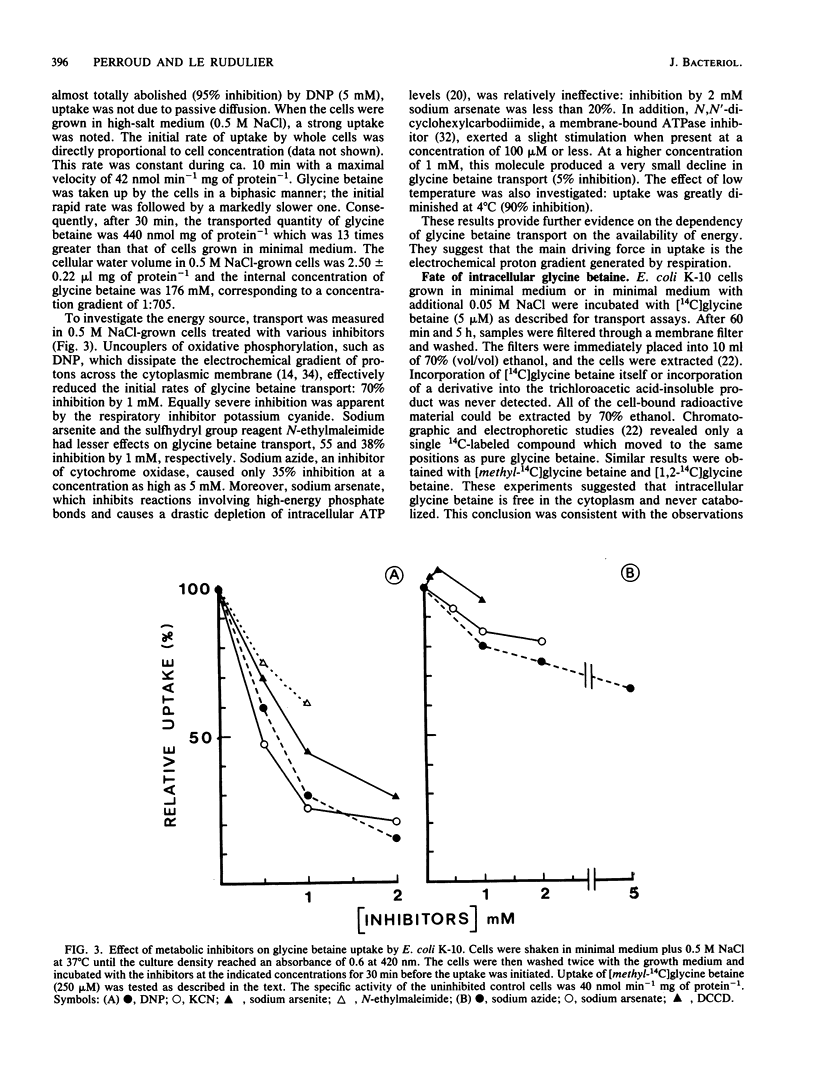
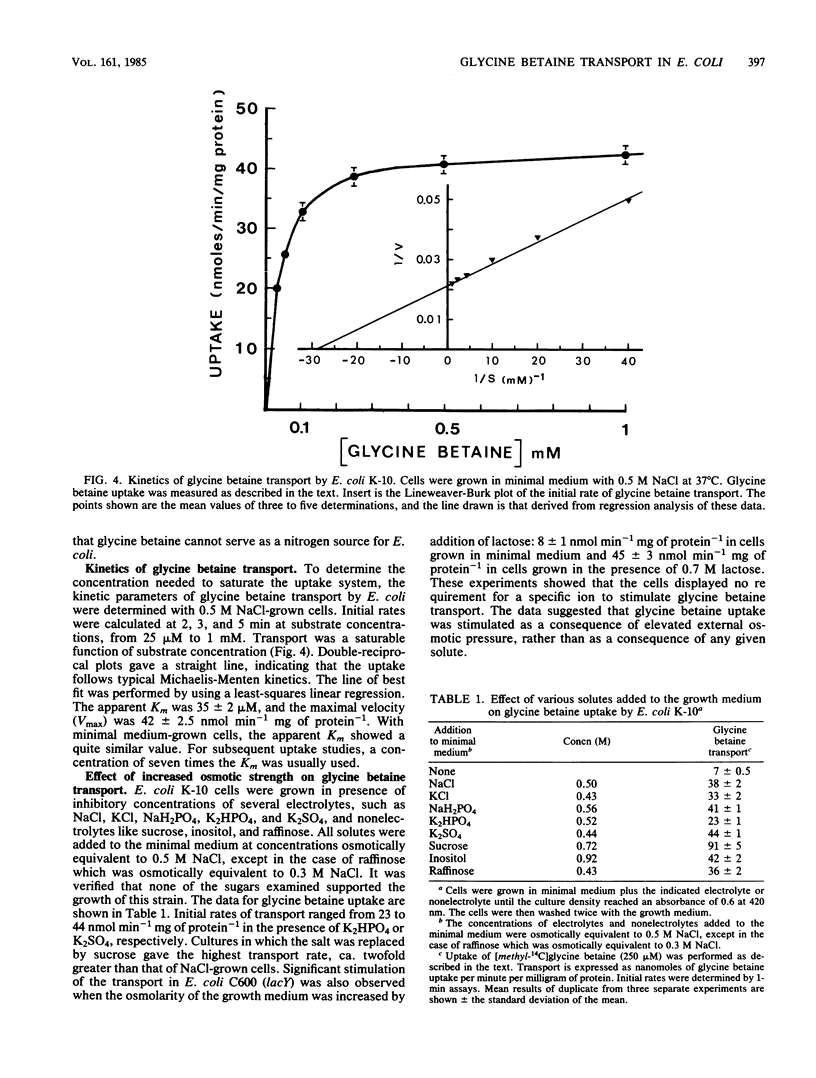
Exogenous glycine betaine highly stimulates the growth rate of various members of the Enterobacteriaceae, including Escherichia coli, in media with high salt concentrations (D. Le Rudulier and L. Bouillard, Appl. Environ. Microbiol. 46:152-159, 1983). In a nitrogen- and carbon-free medium, glycine betaine did not support the growth of E. coli either on low-salt or high-salt media. This molecule was taken up by the cells but was not catabolized. High levels of glycine betaine transport occurred when the cells were grown in media of elevated osmotic strength, whereas relatively low activity was found when the cells were grown in minimal medium. A variety of electrolytes, such as NaCl, KCl, NaH2PO4, K2HPO4, K2SO4, and nonelectrolytes like sucrose, raffinose, and inositol triggered the uptake of glycine betaine. Furthermore, in cells subjected to a sudden osmotic upshock, glycine betaine uptake showed a sixfold stimulation 30 min after the addition of NaCl. Part of this stimulation might be a consequence of protein synthesis. The transport of glycine betaine was energy dependent and occurred against a concentration gradient. 2,4-Dinitrophenol almost totally abolished the glycine betaine uptake. Azide and arsenate exerted only a small inhibition. In addition, N,N'-dicyclohexylcarbodiimide had a very low inhibitory effect at 1 mM. These results indicated that glycine betaine transport is driven by the electrochemical proton gradient. The kinetics of glycine betaine entry followed the Michaelis-Menten relationship, yielding a Km of 35 microM and a Vmax of 42 nmol min-1 mg of protein-1. Glycine betaine transport showed considerable structural specificity. The only potent competitor was proline betaine when added to the assay mixtures at 20-fold the glycine betaine concentration. From these results, it is proposed that E. coli possesses an active and specific glycine betaine transport system which is regulated by the osmotic strength of the growth medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alphen W. V., Lugtenberg B. Influence of osmolarity of the growth medium on the outer membrane protein pattern of Escherichia coli. J Bacteriol. 1977 Aug;131(2):623–630. doi: 10.1128/jb.131.2.623-630.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. R., Menzel R., Wood J. M. Biochemistry and regulation of a second L-proline transport system in Salmonella typhimurium. J Bacteriol. 1980 Mar;141(3):1071–1076. doi: 10.1128/jb.141.3.1071-1076.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Csonka L. N. A third L-proline permease in Salmonella typhimurium which functions in media of elevated osmotic strength. J Bacteriol. 1982 Sep;151(3):1433–1443. doi: 10.1128/jb.151.3.1433-1443.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. Proline over-production results in enhanced osmotolerance in Salmonella typhimurium. Mol Gen Genet. 1981;182(1):82–86. doi: 10.1007/BF00422771. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. The role of proline in osmoregulation in Salmonella typhimurium and Escherichia coli. Basic Life Sci. 1981;18:533–542. doi: 10.1007/978-1-4684-3980-9_32. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the ompB locus in Escherichia coli K-12. J Mol Biol. 1981 Sep 5;151(1):1–15. doi: 10.1016/0022-2836(81)90218-7. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. The ompB locus and the regulation of the major outer membrane porin proteins of Escherichia coli K12. J Mol Biol. 1981 Feb 15;146(1):23–43. doi: 10.1016/0022-2836(81)90364-8. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta S., Matuura K., Imamura S., Misaki H., Horiuti Y. Oxidative pathway of choline to betaine in the soluble fraction prepared from Arthrobacter globiformis. J Biochem. 1977 Jul;82(1):157–163. doi: 10.1093/oxfordjournals.jbchem.a131664. [DOI] [PubMed] [Google Scholar]
- Ikutani Y. Studies of the N-oxides of N,N-dialkylamino acids. I. The synthesis of N,N-dimethyl neutral amino acids and corresponding N-oxides. Bull Chem Soc Jpn. 1968 Jul;41(7):1679–1681. doi: 10.1246/bcsj.41.1679. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Deuel F. Proline uptake by disrupted membrane preparations from Escherichia coli. Arch Biochem Biophys. 1969 Jun;132(1):118–129. doi: 10.1016/0003-9861(69)90343-9. [DOI] [PubMed] [Google Scholar]
- Kawaji H., Mizuno T., Mizushima S. Influence of molecular size and osmolarity of sugars and dextrans on the synthesis of outer membrane proteins O-8 and O-9 of Escherichia coli K-12. J Bacteriol. 1979 Dec;140(3):843–847. doi: 10.1128/jb.140.3.843-847.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P. Osmotic regulation and the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1092–1095. doi: 10.1073/pnas.79.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein W. L., Boyer P. D. Energization of active transport by Escherichia coli. J Biol Chem. 1972 Nov 25;247(22):7257–7265. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laimins L. A., Rhoads D. B., Epstein W. Osmotic control of kdp operon expression in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Jan;78(1):464–468. doi: 10.1073/pnas.78.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rudulier D., Bouillard L. Glycine betaine, an osmotic effector in Klebsiella pneumoniae and other members of the Enterobacteriaceae. Appl Environ Microbiol. 1983 Jul;46(1):152–159. doi: 10.1128/aem.46.1.152-159.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Le Rudulier D., Yang S. S., Csonka L. N. Nitrogen fixation in Klebsiella pneumoniae during osmotic stress. Effect of exogenous proline or a proline overproducing plasmid. Biochim Biophys Acta. 1982 Nov 24;719(2):273–283. doi: 10.1016/0304-4165(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Motojima K., Yamato I., Anraku Y. Proline transport carrier-defective mutants of Escherichia coli K-12: properties and mapping. J Bacteriol. 1978 Oct;136(1):5–9. doi: 10.1128/jb.136.1.5-9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller E., Fahlbusch K., Walther R., Gottschalk G. Formation of N,N-Dimethylglycine, Acetic Acid, and Butyric Acid from Betaine by Eubacterium limosum. Appl Environ Microbiol. 1981 Sep;42(3):439–445. doi: 10.1128/aem.42.3.439-445.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg E., Tietz A., Friedberg I. Effects of salts and ionophores on proline transport in a moderately halopholic halotolerant bacterium. Biochim Biophys Acta. 1980 Feb 15;596(1):118–128. doi: 10.1016/0005-2736(80)90175-3. [DOI] [PubMed] [Google Scholar]
- Ratzkin B., Grabnar M., Roth J. Regulation of the major proline permease gene of Salmonella typhimurium. J Bacteriol. 1978 Feb;133(2):737–743. doi: 10.1128/jb.133.2.737-743.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisin M. P., Kepes A. The membrane ATPase of Escherichia coli. I. Ion dependence and ATP-ADP exchange reaction. Biochim Biophys Acta. 1972 Sep 20;275(3):333–346. doi: 10.1016/0005-2728(72)90214-9. [DOI] [PubMed] [Google Scholar]
- Rowland I., Tristram H. Specificity of the Escherichia coli proline transport system. J Bacteriol. 1975 Sep;123(3):871–877. doi: 10.1128/jb.123.3.871-877.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Postma P. W. The energetics of bacterial active transport. Annu Rev Biochem. 1975;44:523–554. doi: 10.1146/annurev.bi.44.070175.002515. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]


