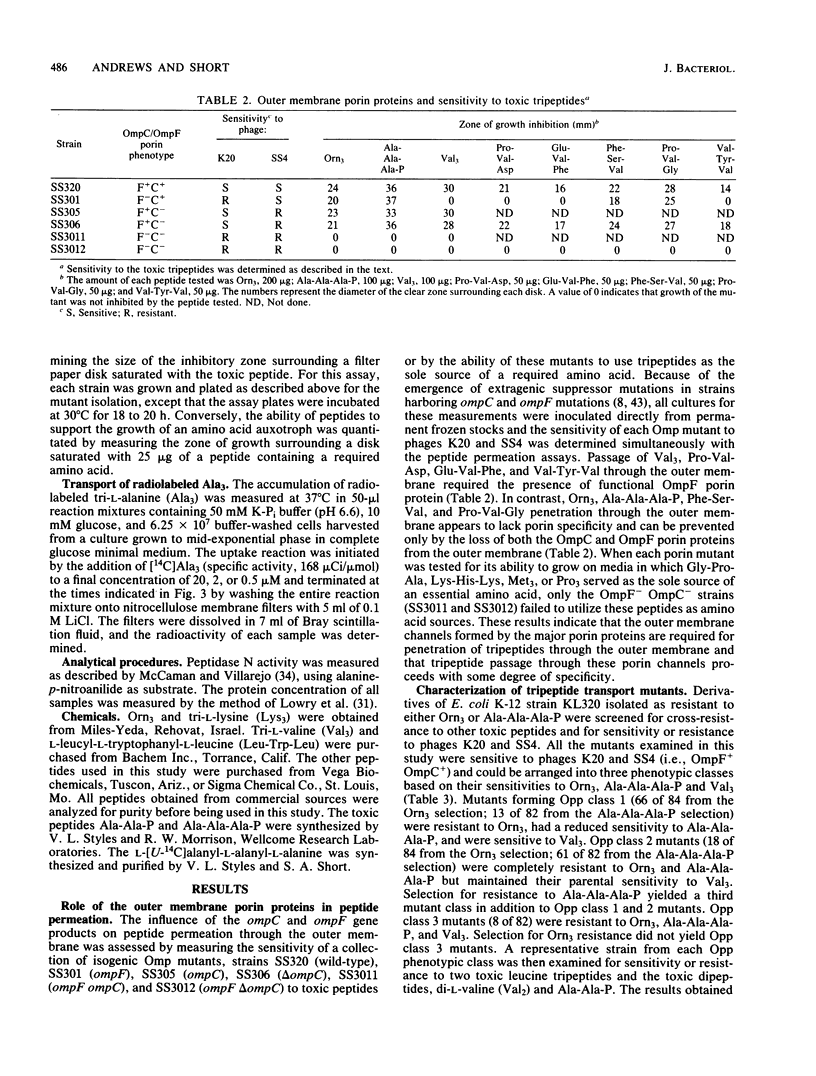
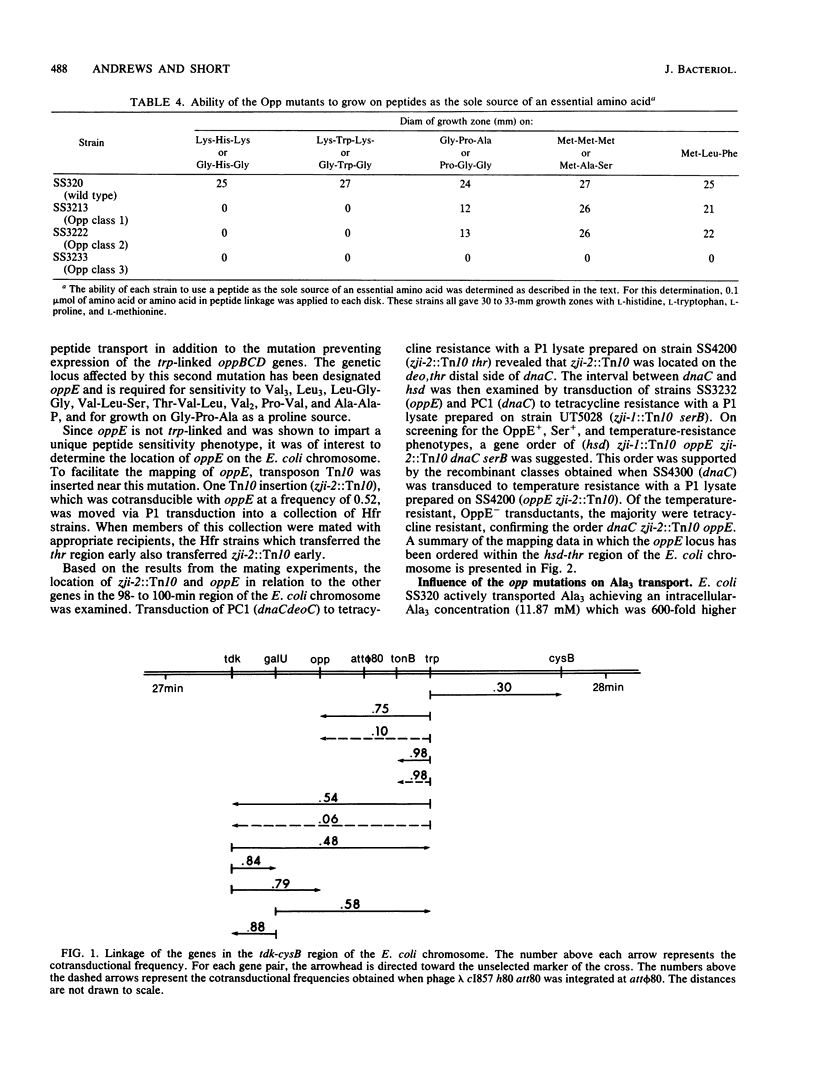
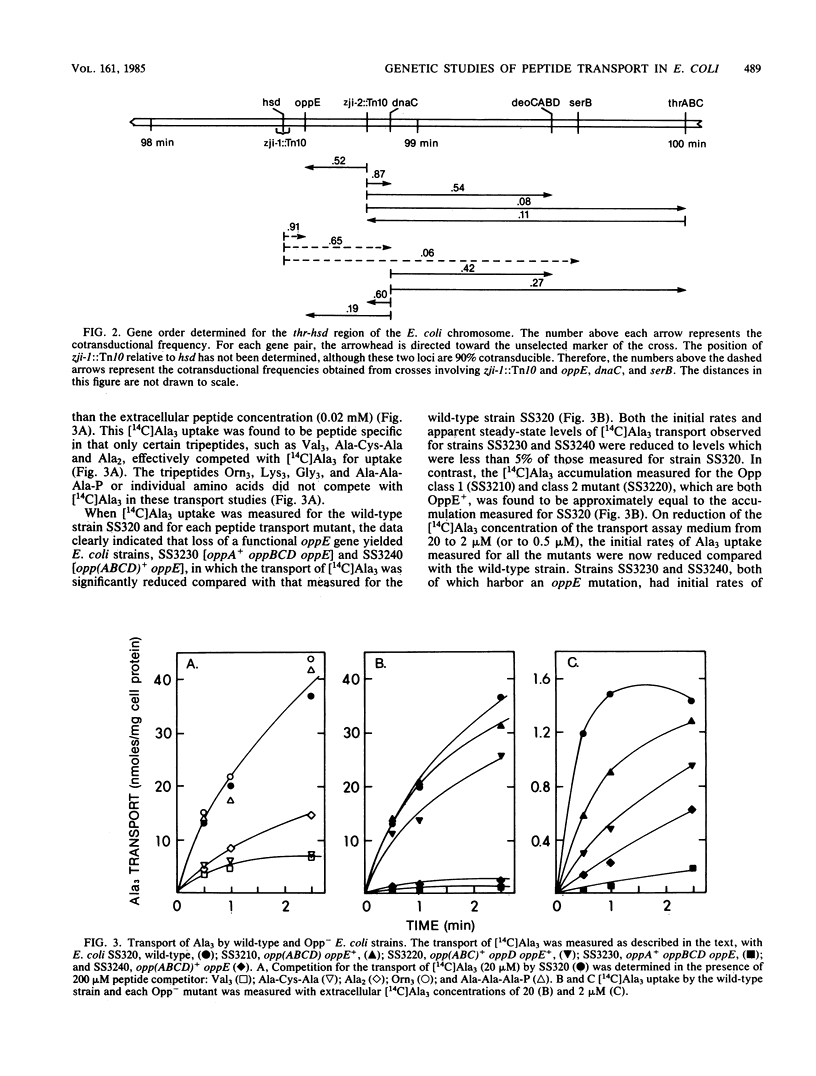
Abstract
The composition of the outer membrane channels formed by the OmpF and OmpC porins is important in peptide permeation, and elimination of these proteins from the Escherichia coli outer membrane results in a cell in which the primary means for peptide permeation through this cell structure has been lost. E. coli peptide transport mutants which harbor defects in genes other than the ompF/ompC genes have been isolated on the basis of their resistance to toxic tripeptides. The genetic defects carried by these oligopeptide permease-negative (Opp-) strains were found to map in two distinct chromosomal locations. One opp locus was trp linked and mapped to the interval between att phi 80 and galU. Complementation studies with F'123 opp derivatives indicated that this peptide transport locus resembles that characterized in Salmonella typhimurium as a tetracistronic operon (B. G. Hogarth and C. F. Higgins, J. Bacteriol. 153:1548-1551, 1983). The second opp locus, which we have designated oppE, was mapped to the interval between dnaC and hsd at 98.5 min on the E. coli chromosome. The differences in peptide utilization, sensitivity and resistance to toxic peptides, and the L-[U-14C]alanyl-L-alanyl-L-alanine transport properties observed with these Opp-E. coli strains demonstrated that the transport systems encoded by the trp-linked opp genes and by the oppE gene(s) have different substrate preferences. Mutants harboring defects in both peptide transport loci defined in this study would not grow on nutritional peptides except for tri-L-methionine, were totally resistant to toxic peptides, and would not actively transport L-[U-14C]alanyl-L-alanyl-L-alanine.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. G., Atherton F. R., Hall M. J., Hassall C. H., Holmes S. W., Lambert R. W., Nisbet L. J., Ringrose P. S. Phosphonopeptides, a new class of synthetic antibacterial agents. Nature. 1978 Mar 2;272(5648):56–58. doi: 10.1038/272056a0. [DOI] [PubMed] [Google Scholar]
- Atherton F. R., Hall M. J., Hassall C. H., Lambert R. W., Lloyd W. J., Lord A. V., Ringrose P. S., Westmacott D. Phosphonopeptides as substrates for peptide transport systems and peptidases of Escherichia coli. Antimicrob Agents Chemother. 1983 Oct;24(4):522–528. doi: 10.1128/aac.24.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton F. R., Hall M. J., Hassall C. H., Lambert R. W., Lloyd W. J., Ringrose P. S. Phosphonopeptides as antibacterial agents: mechanism of action of alaphosphin. Antimicrob Agents Chemother. 1979 May;15(5):696–705. doi: 10.1128/aac.15.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Z., Gilbarg C. Specialized peptide transport system in Escherichia coli. J Bacteriol. 1975 Jun;122(3):1200–1207. doi: 10.1128/jb.122.3.1200-1207.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Z., Gilvarg C. Triornithine-resistant strains of Escherichia coli. Isolation, definition, and genetic studies. J Biol Chem. 1974 Jan 10;249(1):143–148. [PubMed] [Google Scholar]
- Barak Z., Sarid S., Katchalski E. Inhibition of protein biosynthesis in Escherichia coli B by tri-L-ornithine. Eur J Biochem. 1973 Apr;34(2):317–324. doi: 10.1111/j.1432-1033.1973.tb02761.x. [DOI] [PubMed] [Google Scholar]
- Bavoil P., Nikaido H., von Meyenburg K. Pleiotropic transport mutants of Escherichia coli lack porin, a major outer membrane protein. Mol Gen Genet. 1977 Dec 14;158(1):23–33. doi: 10.1007/BF00455116. [DOI] [PubMed] [Google Scholar]
- Cowell J. L. Energetics of glycylglycine transport in Escherichia coli. J Bacteriol. 1974 Oct;120(1):139–146. doi: 10.1128/jb.120.1.139-146.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M., Guardiola J., Lamberti A., Iaccarino M. Escherichia coli K-12 mutants altered in the transport systems for oligo- and dipeptides. J Bacteriol. 1973 Nov;116(2):751–756. doi: 10.1128/jb.116.2.751-756.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M., Levinthal M., Iaccarino M., Guardiola J. Growth inhibition as a consequence of antagonism between related amino acids: effect of valine in Escherichia coli K-12. Microbiol Rev. 1979 Mar;43(1):42–58. doi: 10.1128/mr.43.1.42-58.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decad G. M., Nikaido H. Outer membrane of gram-negative bacteria. XII. Molecular-sieving function of cell wall. J Bacteriol. 1976 Oct;128(1):325–336. doi: 10.1128/jb.128.1.325-336.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M. M., Price M., Higgins C. F. Genetic characterization and molecular cloning of the tripeptide permease (tpp) genes of Salmonella typhimurium. J Bacteriol. 1984 Oct;160(1):122–130. doi: 10.1128/jb.160.1.122-130.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilvarg C., Levin Y. Response of Escherichia coli to ornithyl peptides. J Biol Chem. 1972 Jan 25;247(2):543–549. [PubMed] [Google Scholar]
- Gollop N., Tavori H., Barak Z. Acetohydroxy acid synthase is a target for leucine containing peptide toxicity in Escherichia coli. J Bacteriol. 1982 Jan;149(1):387–390. doi: 10.1128/jb.149.1.387-390.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Phage T6--colicin K receptor and nucleoside transport in Escherichia coli. FEBS Lett. 1976 Nov;70(1):109–112. doi: 10.1016/0014-5793(76)80737-5. [DOI] [PubMed] [Google Scholar]
- Heuzenroeder M. W., Reeves P. Periplasmic maltose-binding protein confers specificity on the outer membrane maltose pore of Escherichia coli. J Bacteriol. 1980 Feb;141(2):431–435. doi: 10.1128/jb.141.2.431-435.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuzenroeder M. W., Reeves P. The tsx protein of Escherichia coli can act as a pore for amino acids. J Bacteriol. 1981 Sep;147(3):1113–1116. doi: 10.1128/jb.147.3.1113-1116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Hardie M. M., Jamieson D., Powell L. M. Genetic map of the opp (Oligopeptide permease) locus of Salmonella typhimurium. J Bacteriol. 1983 Feb;153(2):830–836. doi: 10.1128/jb.153.2.830-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Hardie M. M. Periplasmic protein associated with the oligopeptide permeases of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1983 Sep;155(3):1434–1438. doi: 10.1128/jb.155.3.1434-1438.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S., Igarashi K., Yura T. A deoxythymidine kinase-deficient mutant of Escherichia coli. I. Isolation and some properties. Biochim Biophys Acta. 1967 Aug 22;145(1):41–51. doi: 10.1016/0005-2787(67)90652-1. [DOI] [PubMed] [Google Scholar]
- Hogarth B. G., Higgins C. F. Genetic organization of the oligopeptide permease (opp) locus of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1983 Mar;153(3):1548–1551. doi: 10.1128/jb.153.3.1548-1551.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., Hiraga S., Yura T. A deoxythymidine kinase deficient mutant of Escherichia coli. II. Mapping and transduction studies with phage phi 80. Genetics. 1967 Nov;57(3):643–654. doi: 10.1093/genetics/57.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D. J., Higgins C. F. Anaerobic and leucine-dependent expression of a peptide transport gene in Salmonella typhimurium. J Bacteriol. 1984 Oct;160(1):131–136. doi: 10.1128/jb.160.1.131-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- LEAVITT R. I., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. XI. Valine inhibition of the growth of Escherichia coli strain K-12. J Bacteriol. 1962 Mar;83:624–630. doi: 10.1128/jb.83.3.624-630.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lenny A. B., Margolin P. Locations of the opp and supX genes of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1980 Aug;143(2):747–752. doi: 10.1128/jb.143.2.747-752.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. F. Role of a major outer membrane protein in Escherichia coli. J Bacteriol. 1977 Aug;131(2):631–637. doi: 10.1128/jb.131.2.631-637.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naider F., Becker J. M. Multiplicity of oligopeptide transport systems in Escherichia coli. J Bacteriol. 1975 Jun;122(3):1208–1215. doi: 10.1128/jb.122.3.1208-1215.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J. W., Bell G. Direct determination of the properties of peptide transport systems in Escherichia coli, using a fluorescent-labeling procedure. J Bacteriol. 1979 Jan;137(1):447–455. doi: 10.1128/jb.137.1.447-455.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J. W., Gilvarg C. Size restriction on peptide utilization in Escherichia coli. J Biol Chem. 1968 Dec 10;243(23):6291–6299. [PubMed] [Google Scholar]
- Press R., Glansdorff N., Miner P., De Vries J., Kadner R., Maas W. K. Isolation of transducing particles of phi-80 bacteriophage that carry different regions of the Escherichia coli genome. Proc Natl Acad Sci U S A. 1971 Apr;68(4):795–798. doi: 10.1073/pnas.68.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Identification of three genes controlling production of new outer membrane pore proteins in Escherichia coli K-12. J Bacteriol. 1978 Sep;135(3):1118–1129. doi: 10.1128/jb.135.3.1118-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Szmelcman S., Hofnung M. Maltose transport in Escherichia coli K-12: involvement of the bacteriophage lambda receptor. J Bacteriol. 1975 Oct;124(1):112–118. doi: 10.1128/jb.124.1.112-118.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmelcman S., Schwartz M., Silhavy T. J., Boos W. Maltose transport in Escherichia coli K12. A comparison of transport kinetics in wild-type and lambda-resistant mutants as measured by fluorescence quenching. Eur J Biochem. 1976 May 17;65(1):13–19. doi: 10.1111/j.1432-1033.1976.tb10383.x. [DOI] [PubMed] [Google Scholar]
- Tavori H., Kimmel Y., Barak Z. Toxicity of leucine-containing peptides in Escherichia coli caused by circumvention of leucine transport regulation. J Bacteriol. 1981 May;146(2):676–683. doi: 10.1128/jb.146.2.676-683.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wechsler J. A. Genetic and phenotypic characterization of dnaC mutations. J Bacteriol. 1975 Feb;121(2):594–599. doi: 10.1128/jb.121.2.594-599.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen W., van Seim N., Lugtenberg B. Pores in the outer membrane of Escherichia coli K12: involvement of proteins b and e in the functioning of pores for nucleotides. Mol Gen Genet. 1978 Feb 7;159(1):75–83. doi: 10.1007/BF00401750. [DOI] [PubMed] [Google Scholar]



