Abstract
Gap junctions provide pathways of direct cell to cell communication in the tissues of metazoan animals. Cells joined by gap junctions share their small ions and molecules but can maintain distinctive activities through expression of different macromolecules which are too large to pass through the junctions. The junctional channels are made of a tissue invariant, evolutionarily conserved 16-18 k protein but the formation and maintenance of active coupling also requires one or more connexins, a family of tissue-specific proteins ranging in size from 21 k to 70 k. Junctions can be isolated as complexes containing both types of protein by mild procedures using high pH but the connexins can be removed by detergent, urea and protease treatment without destroying the characteristic junctional-morphology of hexagonally packed channels in the double membrane structures. There is also some evidence for the participation in the complex of tissue-specific proteoglycans which perhaps interact with the tissue-specific connexins and account for specificity of junction formation. Such specificity in mixed cultures leads to the production of communication compartments, groups of cells joined by junctions but separated by reduced trans-boundary coupling from cells in adjacent compartments. Compartmentation also occurs in vivo resulting in specific patterns of junctional communication which have been mapped in most detail in mouse skin. These mapping data and the changes which are associated with abnormal proliferation have lead to new ideas on intercellular control.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. M., Menko A. S., Johnson R. G., Sheppard J. R., Sheridan J. D. Rapid and reversible reduction of junctional permeability in cells infected with a temperature-sensitive mutant of avian sarcoma virus. J Cell Biol. 1981 Nov;91(2 Pt 1):573–578. doi: 10.1083/jcb.91.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargiello T. A., Saez L., Baylies M. K., Gasic G., Young M. W., Spray D. C. The Drosophila clock gene per affects intercellular junctional communication. Nature. 1987 Aug 20;328(6132):686–691. doi: 10.1038/328686a0. [DOI] [PubMed] [Google Scholar]
- Berdan R. C., Gilula N. B. The arthropod gap junction and pseudo-gap junction: isolation and preliminary biochemical analysis. Cell Tissue Res. 1988 Feb;251(2):257–274. doi: 10.1007/BF00215833. [DOI] [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987 Dec;105(6 Pt 1):2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buultjens T. E., Finbow M. E., Lane N. J., Pitts J. D. Tissue and species conservation of the vertebrate and arthropod forms of the low molecular weight (16-18000) proteins of gap junctions. Cell Tissue Res. 1988 Mar;251(3):571–580. doi: 10.1007/BF00214005. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Dahl G., Miller T., Paul D., Voellmy R., Werner R. Expression of functional cell-cell channels from cloned rat liver gap junction complementary DNA. Science. 1987 Jun 5;236(4806):1290–1293. doi: 10.1126/science.3035715. [DOI] [PubMed] [Google Scholar]
- Dermietzel R., Yancey B., Janssen-Timmen U., Traub O., Willecke K., Revel J. P. Simultaneous light and electron microscopic observation of immunolabeled liver 27 KD gap junction protein on ultra-thin cryosections. J Histochem Cytochem. 1987 Mar;35(3):387–392. doi: 10.1177/35.3.3029214. [DOI] [PubMed] [Google Scholar]
- Fentiman I., Taylor-Papadimitriou J., Stoker M. Selective contact-dependent cell communication. Nature. 1976 Dec 23;264(5588):760–762. doi: 10.1038/264760a0. [DOI] [PubMed] [Google Scholar]
- Finbow M. E., Buultjens T. E., John S., Kam E., Meagher L., Pitts J. D. Molecular structure of the gap junctional channel. Ciba Found Symp. 1987;125:92–107. doi: 10.1002/9780470513408.ch6. [DOI] [PubMed] [Google Scholar]
- Finbow M. E., Buultjens T. E., Lane N. J., Shuttleworth J., Pitts J. D. Isolation and characterisation of arthropod gap junctions. EMBO J. 1984 Oct;3(10):2271–2278. doi: 10.1002/j.1460-2075.1984.tb02125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finbow M. E., Shuttleworth J., Hamilton A. E., Pitts J. D. Analysis of vertebrate gap junction protein. EMBO J. 1983;2(9):1479–1486. doi: 10.1002/j.1460-2075.1983.tb01611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finbow M., Yancey S. B., Johnson R., Revel J. P. Independent lines of evidence suggesting a major gap junctional protein with a molecular weight of 26,000. Proc Natl Acad Sci U S A. 1980 Feb;77(2):970–974. doi: 10.1073/pnas.77.2.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher C. Abnormal development in the skin of the pupoid fetus (pf/pf) mutant mouse: abnormal keratinization, recovery of a normal phenotype, and relationship to the repeated epilation (Er/Er) mutant mouse. Curr Top Dev Biol. 1987;22:209–234. doi: 10.1016/s0070-2153(08)60105-2. [DOI] [PubMed] [Google Scholar]
- Furshpan E. J., Potter D. D. Low-resistance junctions between cells in embryos and tissue culture. Curr Top Dev Biol. 1968;3:95–127. doi: 10.1016/s0070-2153(08)60352-x. [DOI] [PubMed] [Google Scholar]
- Goodenough D. A. Bulk isolation of mouse hepatocyte gap junctions. Characterization of the principal protein, connexin. J Cell Biol. 1974 May;61(2):557–563. doi: 10.1083/jcb.61.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenet J. L., Salzgeber B., Tassin M. T. Repeated epilation: a genetic epidermal syndrome in mice. J Hered. 1979 Mar-Apr;70(2):90–94. doi: 10.1093/oxfordjournals.jhered.a109223. [DOI] [PubMed] [Google Scholar]
- Hertzberg E. L. A detergent-independent procedure for the isolation of gap junctions from rat liver. J Biol Chem. 1984 Aug 10;259(15):9936–9943. [PubMed] [Google Scholar]
- Hertzberg E. L., Gilula N. B. Isolation and characterization of gap junctions from rat liver. J Biol Chem. 1979 Mar 25;254(6):2138–2147. [PubMed] [Google Scholar]
- Hertzberg E. L., Spray D. C., Bennett M. V. Reduction of gap junctional conductance by microinjection of antibodies against the 27-kDa liver gap junction polypeptide. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2412–2416. doi: 10.1073/pnas.82.8.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R., Hammer M., Sheridan J., Revel J. P. Gap junction formation between reaggregated Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4536–4540. doi: 10.1073/pnas.71.11.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam E., Melville L., Pitts J. D. Patterns of junctional communication in skin. J Invest Dermatol. 1986 Dec;87(6):748–753. doi: 10.1111/1523-1747.ep12456937. [DOI] [PubMed] [Google Scholar]
- Kam E., Pitts J. D. Effects of the tumour promoter 12-O-tetradecanoylphorbol-13-acetate on junctional communication in intact mouse skin: persistence of homologous communication and increase of epidermal-dermal coupling. Carcinogenesis. 1988 Aug;9(8):1389–1394. doi: 10.1093/carcin/9.8.1389. [DOI] [PubMed] [Google Scholar]
- Kistler J., Christie D., Bullivant S. Homologies between gap junction proteins in lens, heart and liver. Nature. 1988 Feb 25;331(6158):721–723. doi: 10.1038/331721a0. [DOI] [PubMed] [Google Scholar]
- Larsen W. J. Structural diversity of gap junctions. A review. Tissue Cell. 1977;9(3):373–394. doi: 10.1016/0040-8166(77)90001-5. [DOI] [PubMed] [Google Scholar]
- Lo C. W., Gilula N. B. Gap junctional communication in the post-implantation mouse embryo. Cell. 1979 Oct;18(2):411–422. doi: 10.1016/0092-8674(79)90060-6. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Permeability of membrane junctions. Ann N Y Acad Sci. 1966 Jul 14;137(2):441–472. doi: 10.1111/j.1749-6632.1966.tb50175.x. [DOI] [PubMed] [Google Scholar]
- MacDonald C. Genetic complementation in hybrid cells derived from two metabolic co-operative defective mammalian cell lines. Exp Cell Res. 1982 Apr;138(2):303–310. doi: 10.1016/0014-4827(82)90179-3. [DOI] [PubMed] [Google Scholar]
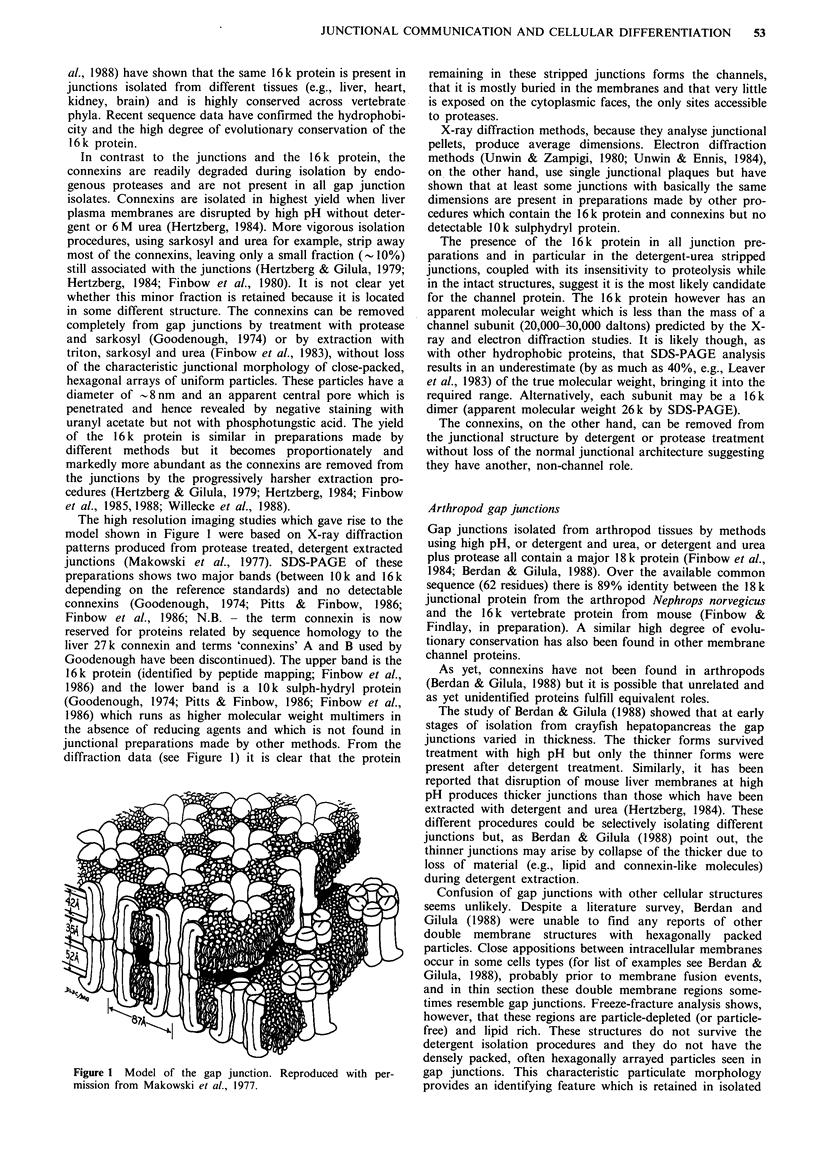
- Makowski L., Caspar D. L., Phillips W. C., Goodenough D. A. Gap junction structures. II. Analysis of the x-ray diffraction data. J Cell Biol. 1977 Aug;74(2):629–645. doi: 10.1083/jcb.74.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson B., Dermietzel R., Teplow D., Traub O., Willecke K., Revel J. P. Two homologous protein components of hepatic gap junctions. Nature. 1987 Oct 22;329(6141):732–734. doi: 10.1038/329732a0. [DOI] [PubMed] [Google Scholar]
- Pitts J. D., Burk R. R. Specificity of junctional communication between animal cells. Nature. 1976 Dec 23;264(5588):762–764. doi: 10.1038/264762a0. [DOI] [PubMed] [Google Scholar]
- Pitts J. D., Finbow M. E. The gap junction. J Cell Sci Suppl. 1986;4:239–266. doi: 10.1242/jcs.1986.supplement_4.15. [DOI] [PubMed] [Google Scholar]
- Pitts J. D., Kam E. Communication compartments in mixed cell cultures. Exp Cell Res. 1985 Feb;156(2):439–449. doi: 10.1016/0014-4827(85)90550-6. [DOI] [PubMed] [Google Scholar]
- Pitts J. D. The role of junctional communication in animal tissues. In Vitro. 1980 Dec;16(12):1049–1056. doi: 10.1007/BF02619255. [DOI] [PubMed] [Google Scholar]
- Pitts J., Kam E., Melville L., Watt F. M. Patterns of junctional communication in animal tissues. Ciba Found Symp. 1987;125:140–153. doi: 10.1002/9780470513408.ch9. [DOI] [PubMed] [Google Scholar]
- Potten C. S. Cell replacement in epidermis (keratopoiesis) via discrete units of proliferation. Int Rev Cytol. 1981;69:271–318. doi: 10.1016/s0074-7696(08)62326-8. [DOI] [PubMed] [Google Scholar]
- Reddy P., Jacquier A. C., Abovich N., Petersen G., Rosbash M. The period clock locus of D. melanogaster codes for a proteoglycan. Cell. 1986 Jul 4;46(1):53–61. doi: 10.1016/0092-8674(86)90859-7. [DOI] [PubMed] [Google Scholar]
- Salomon D., Saurat J. H., Meda P. Cell-to-cell communication within intact human skin. J Clin Invest. 1988 Jul;82(1):248–254. doi: 10.1172/JCI113578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serras F., Buultjens T. E., Finbow M. E. Inhibition of dye-coupling in Patella (Mollusca) embryos by microinjection of antiserum against nephrops (Arthropoda) gap junctions. Exp Cell Res. 1988 Nov;179(1):282–288. doi: 10.1016/0014-4827(88)90367-9. [DOI] [PubMed] [Google Scholar]
- Serras F., van den Biggelaar J. A. Is a mosaic embryo also a mosaic of communication compartments? Dev Biol. 1987 Mar;120(1):132–138. doi: 10.1016/0012-1606(87)90111-4. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Fujita M., Saez J. C., Choi H., Watanabe T., Hertzberg E., Rosenberg L. C., Reid L. M. Proteoglycans and glycosaminoglycans induce gap junction synthesis and function in primary liver cultures. J Cell Biol. 1987 Jul;105(1):541–551. doi: 10.1083/jcb.105.1.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B. R., Siliciano J. D., Mooseker M. S., Goodenough D. A. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986 Sep;103(3):755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub O., Janssen-Timmen U., Drüge P. M., Dermietzel R., Willecke K. Immunological properties of gap junction protein from mouse liver. J Cell Biochem. 1982;19(1):27–44. doi: 10.1002/jcb.240190104. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Ennis P. D. Two configurations of a channel-forming membrane protein. Nature. 1984 Feb 16;307(5952):609–613. doi: 10.1038/307609a0. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Zampighi G. Structure of the junction between communicating cells. Nature. 1980 Feb 7;283(5747):545–549. doi: 10.1038/283545a0. [DOI] [PubMed] [Google Scholar]
- Warner A. E., Guthrie S. C., Gilula N. B. Antibodies to gap-junctional protein selectively disrupt junctional communication in the early amphibian embryo. Nature. 1984 Sep 13;311(5982):127–131. doi: 10.1038/311127a0. [DOI] [PubMed] [Google Scholar]
- Warner A. E., Lawrence P. A. Permeability of gap junctions at the segmental border in insect epidermis. Cell. 1982 Feb;28(2):243–252. doi: 10.1016/0092-8674(82)90342-7. [DOI] [PubMed] [Google Scholar]
- Yotti L. P., Chang C. C., Trosko J. E. Elimination of metabolic cooperation in Chinese hamster cells by a tumor promoter. Science. 1979 Nov 30;206(4422):1089–1091. doi: 10.1126/science.493994. [DOI] [PubMed] [Google Scholar]
- Zimmer D. B., Green C. R., Evans W. H., Gilula N. B. Topological analysis of the major protein in isolated intact rat liver gap junctions and gap junction-derived single membrane structures. J Biol Chem. 1987 Jun 5;262(16):7751–7763. [PubMed] [Google Scholar]



