Abstract
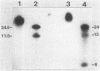
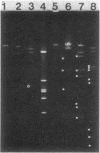
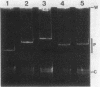
The broad-host-range plasmid RP4 and its derivative R68.45 were transferred to Myxococcus xanthus DK101 and DZ1; RP4 was maintained integrated in the chromosome. Loss of plasmid markers occurred during the growth of the transconjugants, which could be prevented by selective pressure with oxytetracycline. The integrated plasmid was transferred back to Escherichia coli often as RP4-prime plasmids carrying various segments of the M. xanthus chromosome. It also mediated chromosomal transfer between M. xanthus strains.
Full text
PDF

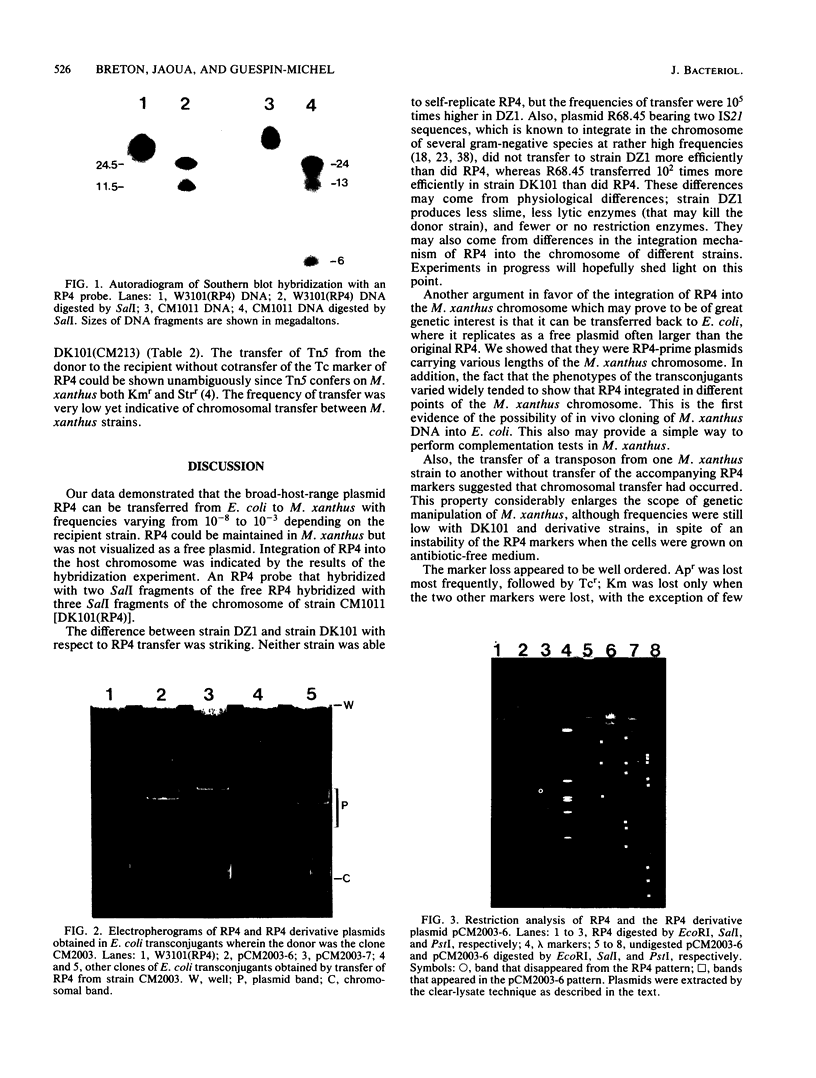


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avery L., Kaiser D. In situ transposon replacement and isolation of a spontaneous tandem genetic duplication. Mol Gen Genet. 1983;191(1):99–109. doi: 10.1007/BF00330896. [DOI] [PubMed] [Google Scholar]
- Barrett J. T., Rhodes C. S., Ferber D. M., Jenkins B., Kuhl S. A., Ely B. Construction of a genetic map for Caulobacter crescentus. J Bacteriol. 1982 Mar;149(3):889–896. doi: 10.1128/jb.149.3.889-896.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck Y., Coetzee W. F., Coetzee J. N. In vitro-constructed RP4-prime plasmids mediate orientated mobilization of the Proteus morganii chromosome. J Gen Microbiol. 1982 Jun;128(6):1163–1169. doi: 10.1099/00221287-128-6-1163. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Multiple molecular species of circular R-factor DNA isolated from Escherichia coli. Nature. 1969 Dec 27;224(5226):1273–1277. doi: 10.1038/2241273a0. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Host ranges of R factors. J Gen Microbiol. 1972 May;70(3):453–460. doi: 10.1099/00221287-70-3-453. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Haas D., Holloway B. W. R factor variants with enhanced sex factor activity in Pseudomonas aeruginosa. Mol Gen Genet. 1976 Mar 30;144(3):243–251. doi: 10.1007/BF00341722. [DOI] [PubMed] [Google Scholar]
- Haas D., Riess G. Spontaneous deletions of the chromosome-mobilizing plasmid R68.45 in Pseudomonas aeruginosa PAO. Plasmid. 1983 Jan;9(1):42–52. doi: 10.1016/0147-619x(83)90030-6. [DOI] [PubMed] [Google Scholar]
- Harayama S., Tsuda M., Iino T. High frequency mobilization of the chromosome of Escherichia coli by a mutant of plasmid RP4 temperature-sensitive for maintenance. Mol Gen Genet. 1980;180(1):47–56. doi: 10.1007/BF00267351. [DOI] [PubMed] [Google Scholar]
- Hassan D. M., Brevet J. Absence of cis-acting transposition immunity with Tn7. Plasmid. 1983 Jul;10(1):31–44. doi: 10.1016/0147-619x(83)90055-0. [DOI] [PubMed] [Google Scholar]
- Holloway B. W. Isolation and characterization of an R' plasmid in Pseudomonas aeruginosa. J Bacteriol. 1978 Mar;133(3):1078–1082. doi: 10.1128/jb.133.3.1078-1082.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W. Plasmids that mobilize bacterial chromosome. Plasmid. 1979 Jan;2(1):1–19. doi: 10.1016/0147-619x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- Julliot J. S., Boistard P. Use of RP4-prime plasmids constructed in vitro to promote a polarized transfer of the chromosome in Escherichia coli and Rhizobium meliloti. Mol Gen Genet. 1979 Jun 20;173(3):289–298. doi: 10.1007/BF00268639. [DOI] [PubMed] [Google Scholar]
- Kaiser D., Manoil C., Dworkin M. Myxobacteria: cell interactions, genetics, and development. Annu Rev Microbiol. 1979;33:595–639. doi: 10.1146/annurev.mi.33.100179.003115. [DOI] [PubMed] [Google Scholar]
- Lejeune P., Mergeay M., Van Gijsegem F., Faelen M., Gerits J., Toussaint A. Chromosome transfer and R-prime plasmid formation mediated by plasmid pULB113 (RP4::mini-Mu) in Alcaligenes eutrophus CH34 and Pseudomonas fluorescens 6.2. J Bacteriol. 1983 Sep;155(3):1015–1026. doi: 10.1128/jb.155.3.1015-1026.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann K., Clarke C. H. Plasmid-mediated UV-protection in Myxococcus xanthus. Mol Gen Genet. 1981;182(1):137–142. doi: 10.1007/BF00422780. [DOI] [PubMed] [Google Scholar]
- Moore A. T., Nayudu M., Holloway B. W. Genetic mapping in Methylophilus methylotrophus AS1. J Gen Microbiol. 1983 Mar;129(3):785–799. doi: 10.1099/00221287-129-3-785. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor K. A., Zusman D. R. Coliphage P1-mediated transduction of cloned DNA from Escherichia coli to Myxococcus xanthus: use for complementation and recombinational analyses. J Bacteriol. 1983 Jul;155(1):317–329. doi: 10.1128/jb.155.1.317-329.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi N., Izaki K., Takahashi H. A macrocyclic antibiotic M-230B produced by Myxococcus xanthus. Isolation and characterization. J Antibiot (Tokyo) 1984 Jan;37(1):13–19. doi: 10.7164/antibiotics.37.13. [DOI] [PubMed] [Google Scholar]
- Parish J. H. Transfer of drug resistance to myxococcus from bacteria carrying drug-resistance factors. J Gen Microbiol. 1975 Apr;87(2):198–210. doi: 10.1099/00221287-87-2-198. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saunders J. R., Grinsted J. Properties of RP4, an R factor which originated in Pseudomonas aeruginosa S8. J Bacteriol. 1972 Nov;112(2):690–696. doi: 10.1128/jb.112.2.690-696.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab H., Saurugger P. N., Lafferty R. M. Occurrence of deletion plasmids at high rates after conjugative transfer of the plasmids RP4 and RK2 from Escherichia coli to Alcaligenes eutrophus H16. Arch Microbiol. 1983 Nov;136(2):140–146. doi: 10.1007/BF00404789. [DOI] [PubMed] [Google Scholar]
- Shimkets L. J., Gill R. E., Kaiser D. Developmental cell interactions in Myxococcus xanthus and the spoC locus. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1406–1410. doi: 10.1073/pnas.80.5.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner K. J., Vivian A. RP4-mediated conjugation in Acinetobacter calcoaceticus. J Gen Microbiol. 1976 Apr;93(2):355–360. doi: 10.1099/00221287-93-2-355. [DOI] [PubMed] [Google Scholar]
- Van Gijsegem F., Toussaint A. Chromosome transfer and R-prime formation by an RP4::mini-Mu derivative in Escherichia coli, Salmonella typhimurium, Klebsiella pneumoniae, and Proteus mirabilis. Plasmid. 1982 Jan;7(1):30–44. doi: 10.1016/0147-619x(82)90024-5. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Crowther C., Holloway B. W. The insertion sequence IS21 of R68.45 and the molecular basis for mobilization of the bacterial chromosome. Plasmid. 1981 Jul;6(1):30–52. doi: 10.1016/0147-619x(81)90052-4. [DOI] [PubMed] [Google Scholar]
- Zafriri D., Rosenberg E., Mirelman D. Mode of action of Myxococcus xanthus antibiotic TA. Antimicrob Agents Chemother. 1981 Feb;19(2):349–351. doi: 10.1128/aac.19.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusman D. R., Krotoski D. M., Cumsky M. Chromosome replication in Myxococcus xanthus. J Bacteriol. 1978 Jan;133(1):122–129. doi: 10.1128/jb.133.1.122-129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]