Abstract
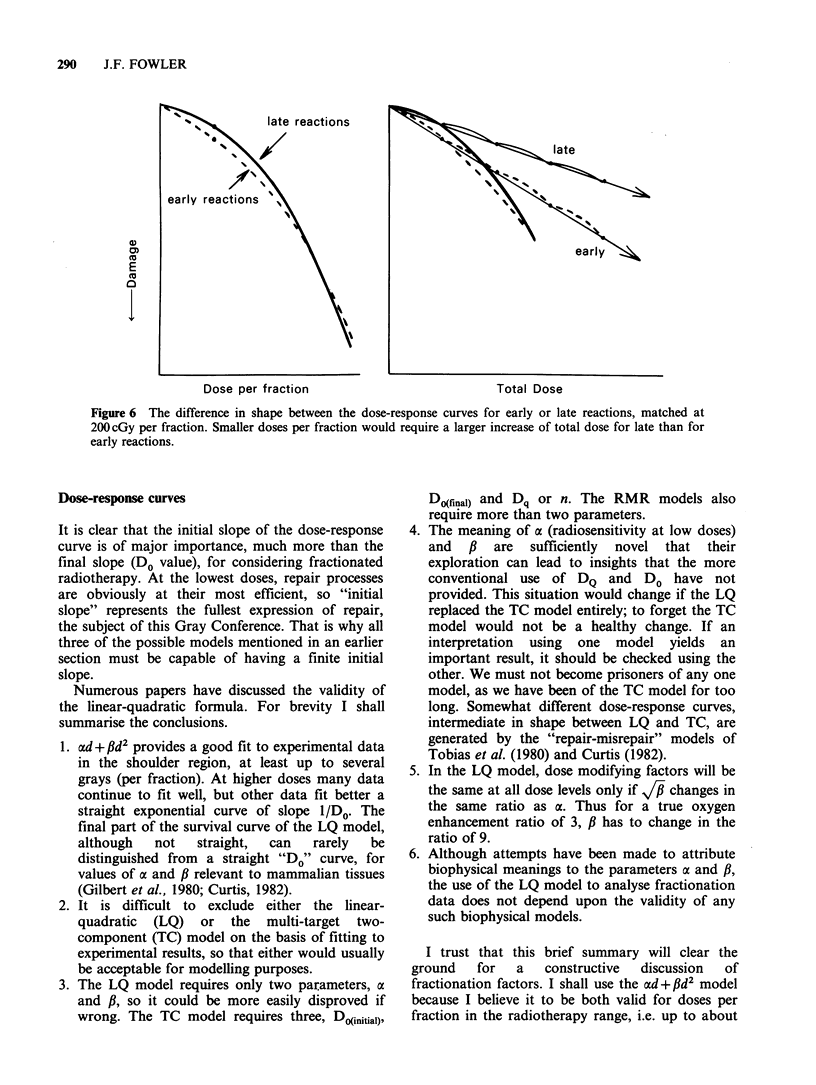
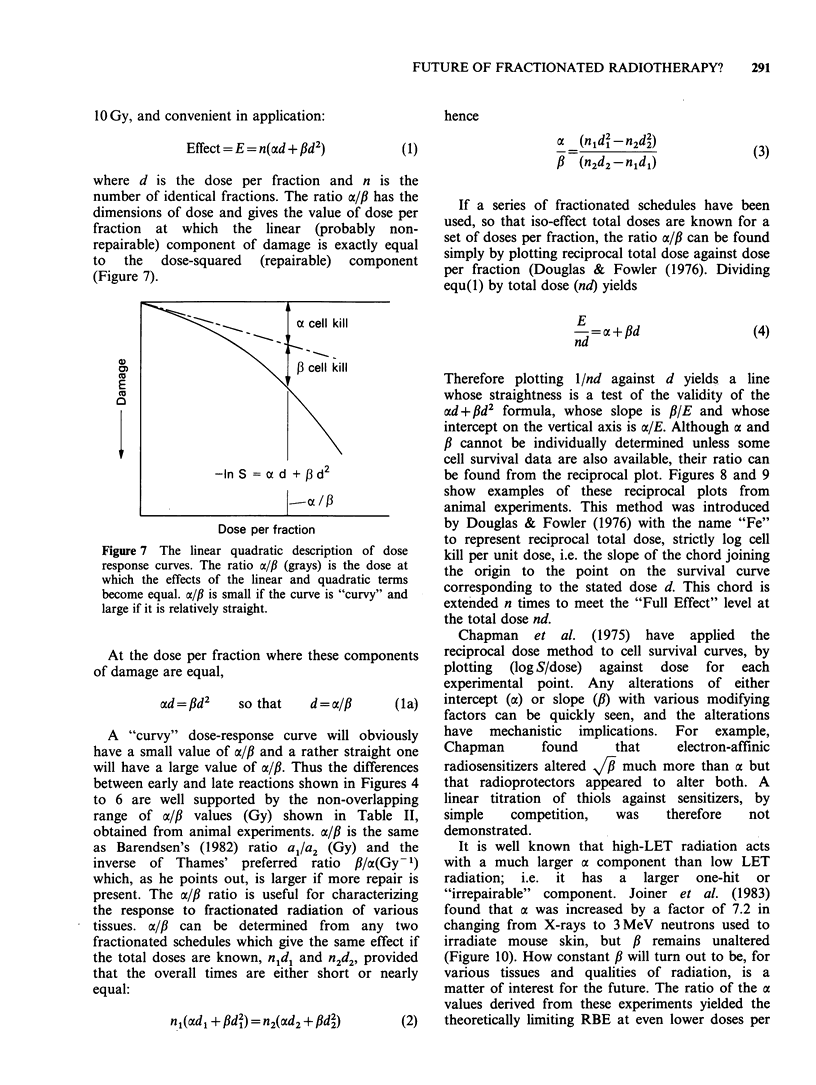
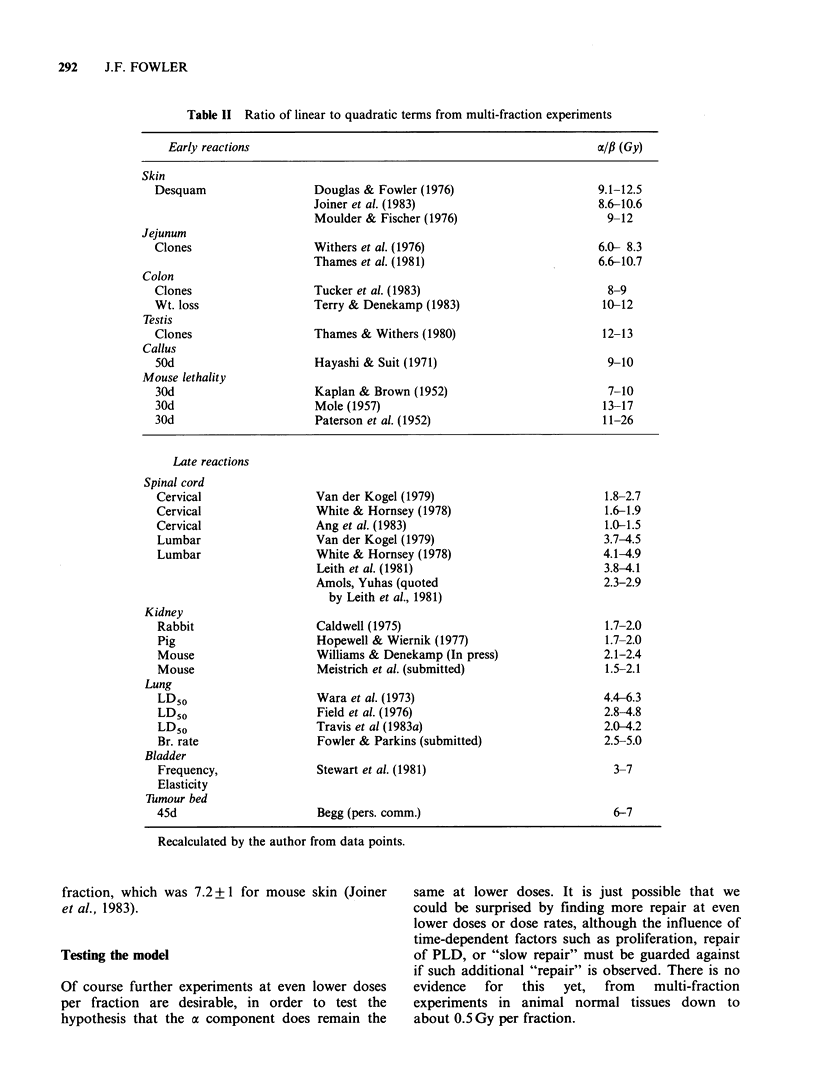
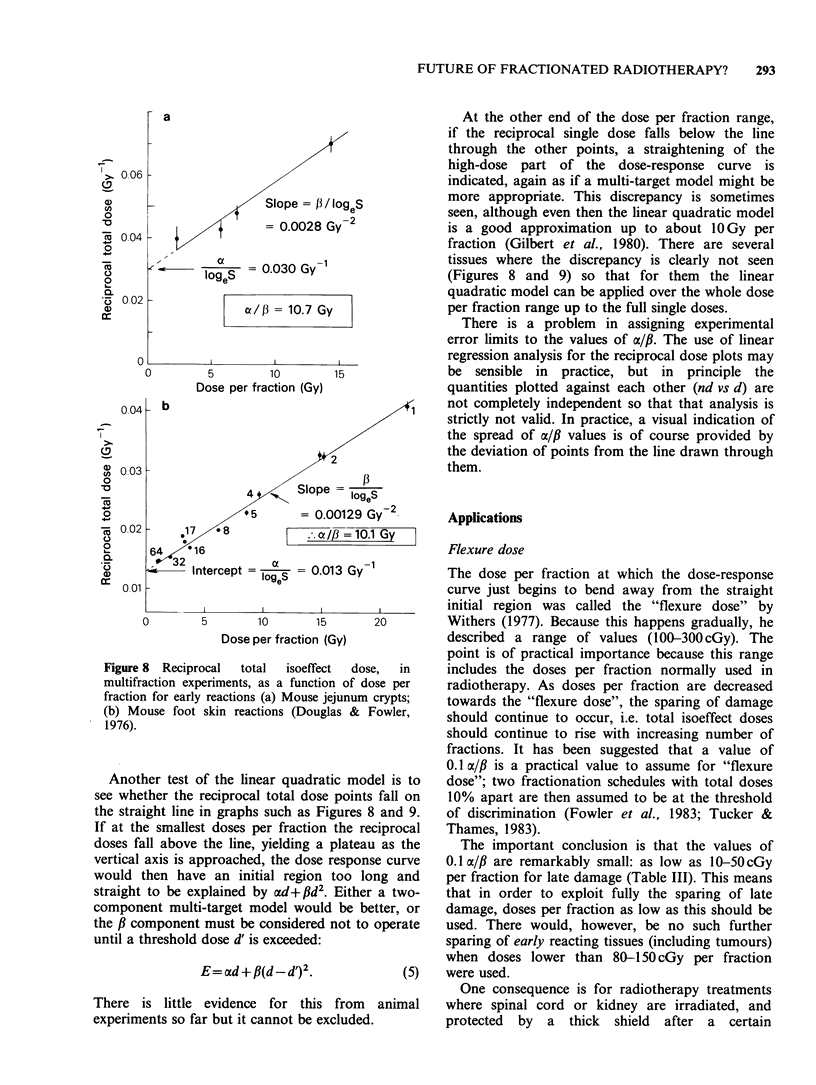
Models for predicting the total dose required to produce tolerable normal-tissue injury are becoming less empirical, more realistic, and more specific for different tissue reactions. The trend can be seen by the progression from the "cube root law", through Strandqvist's slope of 0.22, to NSD, TDF and CRE which have separate time and fraction number exponents, to the even better approximations which are now available. The dose-response formulae that can be used, with statistical legitimacy, to define the effect of fraction size (and number) include (1) the linear quadratic(LQ) model; (2) the two-component (TC) multi-target model; and (3) repair - misrepair models. The LQ model offers considerable convenience and requires only two parameters to be determined. The use of a new model often provides fresh insights. The LQ model has emphasized the difference between late and early normal-tissue dependence on dose per fraction which was first shown by exponents greater than the NSD slope of 0.24. Exponents of overall time, e.g. T0.11, yield the wrong shape of time curve, suggesting that most proliferation occurs early, although it really occurs after a delay depending on the turnover time of the tissue. The principles of better time factors are well known but actual values for human tissues are not well determined. Fortunately the time factors are usually small, especially for late reactions. Improved clinical results are being sought by hyperfractionation, by accelerated fractionation, or by continuous low dose rate irradiation as in interstitial implants. New clinical trials are investigating these approaches, which have been suggested by the accumulation of radiobiological data.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ang K. K., van der Kogel A. J., van der Schueren E. The effect of small radiation doses on the rat spinal cord: the concept of partial tolerance. Int J Radiat Oncol Biol Phys. 1983 Oct;9(10):1487–1491. doi: 10.1016/0360-3016(83)90323-1. [DOI] [PubMed] [Google Scholar]
- Barendsen G. W. Dose fractionation, dose rate and iso-effect relationships for normal tissue responses. Int J Radiat Oncol Biol Phys. 1982 Nov;8(11):1981–1997. doi: 10.1016/0360-3016(82)90459-x. [DOI] [PubMed] [Google Scholar]
- Bates T. D., Peters L. J. Letter: Dangers of the clinical use of the NSD formula for small fraction numbers. Br J Radiol. 1975 Sep;48(573):773–773. doi: 10.1259/0007-1285-48-573-773-b. [DOI] [PubMed] [Google Scholar]
- Chapman J. D., Gillespie C. J., Reuvers A. P., Dugle D. L. The inactivation of Chinese hamster cells by x rays: the effects of chemical modifiers on single- and double-events. Radiat Res. 1975 Nov;64(2):365–375. [PubMed] [Google Scholar]
- Cohen L. Theoretical "iso-survival" formulae for fractionated radiation therapy. Br J Radiol. 1968 Jul;41(487):522–528. doi: 10.1259/0007-1285-41-487-522. [DOI] [PubMed] [Google Scholar]
- Denekamp J. Changes in the rate of repopulation during multifraction irradiation of mouse skin. Br J Radiol. 1973 May;46(545):381–387. doi: 10.1259/0007-1285-46-545-381. [DOI] [PubMed] [Google Scholar]
- Denekamp J., Stewart F. A. Evidence for repair capacity in mouse tumors relative to skin. Int J Radiat Oncol Biol Phys. 1979 Nov-Dec;5(11-12):2003–2010. doi: 10.1016/0360-3016(79)90952-0. [DOI] [PubMed] [Google Scholar]
- Douglas B. G., Fowler J. F. The effect of multiple small doses of x rays on skin reactions in the mouse and a basic interpretation. Radiat Res. 1976 May;66(2):401–426. [PubMed] [Google Scholar]
- Douglas B. G. Implications of the quadratic cell survival curve and human skin radiation "tolerance doses" on fractionation and superfractionation dose selection. Int J Radiat Oncol Biol Phys. 1982 Jul;8(7):1135–1142. doi: 10.1016/0360-3016(82)90061-x. [DOI] [PubMed] [Google Scholar]
- Ellis F. Dose, time and fractionation: a clinical hypothesis. Clin Radiol. 1969 Jan;20(1):1–7. doi: 10.1016/s0009-9260(69)80043-7. [DOI] [PubMed] [Google Scholar]
- FOWLER J. F., STERN B. E. Fractionation and dose-rate. II. Dose-time relationships in radiotherapy and the validity of cell survival curve models. Br J Radiol. 1963 Mar;36:163–173. doi: 10.1259/0007-1285-36-423-163. [DOI] [PubMed] [Google Scholar]
- Field S. B., Hornsey S., Kutsutani Y. Effects of fractionated irradiation on mouse lung and a phenomenon of slow repair. Br J Radiol. 1976 Aug;49(584):700–707. doi: 10.1259/0007-1285-49-584-700. [DOI] [PubMed] [Google Scholar]
- Fowler J. F., Denekamp J., Delapeyre C., Harris S. R., Sheldon P. W. Skin reactions in mice after multifraction x-irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1974 Mar;25(3):213–223. doi: 10.1080/09553007414550271. [DOI] [PubMed] [Google Scholar]
- Fowler J. F. Dose response curves for organ function or cell survival. Br J Radiol. 1983 Jul;56(667):497–500. doi: 10.1259/0007-1285-56-667-497. [DOI] [PubMed] [Google Scholar]
- Fowler J. F. Experimental animal results relating to time-dose relationships in radiotherapy and the "ret" concept. Br J Radiol. 1971 Feb;44(518):81–90. doi: 10.1259/0007-1285-44-518-81. [DOI] [PubMed] [Google Scholar]
- Fowler J. F., Joiner M. C., Williams M. V. Low doses per fraction in radiotherapy: a definition for "flexure dose". Br J Radiol. 1983 Aug;56(668):599–601. doi: 10.1259/0007-1285-56-668-599. [DOI] [PubMed] [Google Scholar]
- Gilbert C. W., Hendry J. H., Major D. The approximation in the formulation for survival S=exp-(alpha D + beta D2). Int J Radiat Biol Relat Stud Phys Chem Med. 1980 Apr;37(4):469–471. doi: 10.1080/09553008014550571. [DOI] [PubMed] [Google Scholar]
- Glatstein E. Alterations in rubidium-86 extraction in normal mouse tissues after irradiation. An estimate of long-term blood flow changes in kidney, lung, liver, skin and muscle. Radiat Res. 1973 Jan;53(1):88–101. [PubMed] [Google Scholar]
- Hahn G. M., Bagshaw M. A. Serum concentration: effects on cycle and x-ray sensitivity of mammalian cells. Science. 1966 Jan 28;151(3709):459–461. doi: 10.1126/science.151.3709.459. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Suit H. D. Effect of fractionation of radiation dose on callus formation at site of fracture. Radiology. 1971 Oct;101(1):181–186. doi: 10.1148/101.1.181. [DOI] [PubMed] [Google Scholar]
- Hornsey S., Myers R., Coultas P. G., Rogers M. A., White A. Turnover of proliferative cells in the spinal cord after X irradiation and its relation to time-dependent repair of radiation damage. Br J Radiol. 1981 Dec;54(648):1081–1085. doi: 10.1259/0007-1285-54-648-1081. [DOI] [PubMed] [Google Scholar]
- Joiner M. C., Maughan R. L., Fowler J. F., Denekamp J. The RBE for mouse skin irradiated with 3-MeV neutrons: single and fractionated doses. Radiat Res. 1983 Jul;95(1):130–141. [PubMed] [Google Scholar]
- KAPLAN H. S., BROWN M. B. Mortality of mice after total-body irradiation as influenced by alterations in total dose, fractionation, and periodicity of treatment. J Natl Cancer Inst. 1952 Feb;12(4):765–775. [PubMed] [Google Scholar]
- Kirk J., Gray W. M., Watson E. R. Cumulative radiation effect. I. Fractionated treatment regimes. Clin Radiol. 1971 Apr;22(2):145–155. doi: 10.1016/s0009-9260(71)80044-2. [DOI] [PubMed] [Google Scholar]
- Leith J. T., DeWyngaert J. K., Glicksman A. S. Radiation myelopathy in the rat: an interpretation of dose effect relationships. Int J Radiat Oncol Biol Phys. 1981 Dec;7(12):1673–1677. doi: 10.1016/0360-3016(81)90191-7. [DOI] [PubMed] [Google Scholar]
- MOLE R. H. Quantitative observations on recovery from whole body irradiation in mice. II. Recovery during and after daily irradiation. Br J Radiol. 1957 Jan;30(349):40–46. doi: 10.1259/0007-1285-30-349-40. [DOI] [PubMed] [Google Scholar]
- Moulder J. E., Fischer J. J. Radiation reaction of rat skin. The role of the number of fractions and the overall treatment time. Cancer. 1976 Jun;37(6):2762–2767. doi: 10.1002/1097-0142(197606)37:6<2762::aid-cncr2820370629>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Orton C. G., Ellis F. A simplification in the use of the NSD concept in practical radiotherapy. Br J Radiol. 1973 Jul;46(547):529–537. doi: 10.1259/0007-1285-46-547-529. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Bova F. J., Million R. R. A re-evaluation of split-course technique for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1980 Dec;6(12):1645–1652. doi: 10.1016/0360-3016(80)90246-1. [DOI] [PubMed] [Google Scholar]
- Sinclair W. K., Morton R. A. X-ray sensitivity during the cell generation cycle of cultured Chinese hamster cells. Radiat Res. 1966 Nov;29(3):450–474. [PubMed] [Google Scholar]
- Singh K. Two regimes with the same TDF but differing morbidity used in the treatment of stage III carcinoma of the cervix. Br J Radiol. 1978 May;51(605):357–362. doi: 10.1259/0007-1285-51-605-357. [DOI] [PubMed] [Google Scholar]
- Stewart F. A., Randhawa V. S., Michael B. D., Denekamp J. Repair during fractionated irradiation of the mouse bladder. Br J Radiol. 1981 Sep;54(645):799–804. doi: 10.1259/0007-1285-54-645-799. [DOI] [PubMed] [Google Scholar]
- Svoboda V. H. Further experience with radiotherapy by multiple daily sessions. Br J Radiol. 1978 May;51(605):363–369. doi: 10.1259/0007-1285-51-605-363. [DOI] [PubMed] [Google Scholar]
- Thames H. D., Jr, Peters L. J., Withers H. R., Fletcher G. H. Accelerated fractionation vs hyperfractionation: rationales for several treatments per day. Int J Radiat Oncol Biol Phys. 1983 Feb;9(2):127–138. doi: 10.1016/0360-3016(83)90089-5. [DOI] [PubMed] [Google Scholar]
- Thames H. D., Jr, Withers H. R., Peters L. J., Fletcher G. H. Changes in early and late radiation responses with altered dose fractionation: implications for dose-survival relationships. Int J Radiat Oncol Biol Phys. 1982 Feb;8(2):219–226. doi: 10.1016/0360-3016(82)90517-x. [DOI] [PubMed] [Google Scholar]
- Thames H. D., Jr, Withers H. R. Test of equal effect per fraction and estimation of initial clonogen number in microcolony assays of survival after fractionated irradiation. Br J Radiol. 1980 Nov;53(635):1071–1077. doi: 10.1259/0007-1285-53-635-1071. [DOI] [PubMed] [Google Scholar]
- Thames H. D., Jr, Withers R., Mason K. A., Reid B. O. Dose-survival characteristics of mouse jejunal crypt cells. Int J Radiat Oncol Biol Phys. 1981 Nov;7(11):1591–1597. doi: 10.1016/0360-3016(81)90091-2. [DOI] [PubMed] [Google Scholar]
- Travis E. L., Down J. D. Repair in mouse lung after split doses of X rays. Radiat Res. 1981 Jul;87(1):166–174. [PubMed] [Google Scholar]
- Travis E. L., Parkins C. S., Down J. D., Fowler J. F., Maughan R. L. Is there a loss of repair capacity in mouse lungs with increasing numbers of dose fractions? Int J Radiat Oncol Biol Phys. 1983 May;9(5):691–699. doi: 10.1016/0360-3016(83)90236-5. [DOI] [PubMed] [Google Scholar]
- Travis E. L., Parkins C. S., Down J. D., Fowler J. F., Thames H. D., Jr Repair in mouse lung between multiple small doses of X rays. Radiat Res. 1983 May;94(2):326–339. [PubMed] [Google Scholar]
- Trott K. R. Experimental results and clinical implications of the four R's in fractionated radiotherapy. Radiat Environ Biophys. 1982;20(3):159–170. doi: 10.1007/BF01325465. [DOI] [PubMed] [Google Scholar]
- Tubiana M. L.H. Gray Medal lecture: cell kinetics and radiation oncology. Int J Radiat Oncol Biol Phys. 1982 Sep;8(9):1471–1489. doi: 10.1016/0360-3016(82)90607-1. [DOI] [PubMed] [Google Scholar]
- Tucker S. L., Thames H. D., Jr Flexure dose: the low-dose limit of effective fractionation. Int J Radiat Oncol Biol Phys. 1983 Sep;9(9):1373–1383. doi: 10.1016/0360-3016(83)90270-5. [DOI] [PubMed] [Google Scholar]
- Tucker S. L., Withers H. R., Mason K. A., Thames H. D., Jr A dose-surviving fraction curve for mouse colonic mucosa. Eur J Cancer Clin Oncol. 1983 Mar;19(3):433–437. doi: 10.1016/0277-5379(83)90143-8. [DOI] [PubMed] [Google Scholar]
- Wara W. M., Phillips T. L., Margolis L. W., Smith V. Radiation pneumonitis: a new approach to the derivation of time-dose factors. Cancer. 1973 Sep;32(3):547–552. doi: 10.1002/1097-0142(197309)32:3<547::aid-cncr2820320306>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Wheldon T. E., Michalowski A. S., Kirk J. The effect of irradiation on function in self-renewing normal tissues with differing proliferative organisation. Br J Radiol. 1982 Oct;55(658):759–766. doi: 10.1259/0007-1285-55-658-759. [DOI] [PubMed] [Google Scholar]
- White A., Hornsey S. Radiation damage to the rat spinal cord: the effect of single and fractionated doses of X rays. Br J Radiol. 1978 Jul;51(607):515–523. doi: 10.1259/0007-1285-51-607-515. [DOI] [PubMed] [Google Scholar]
- Williams M. V., Denekamp J. Sequential functional testing of radiation-induced renal damage in the mouse. Radiat Res. 1983 May;94(2):305–317. [PubMed] [Google Scholar]
- Withers H. R., Reid B. O., Hussey D. H. Response of mouse jejunum to multifraction radiation. Int J Radiat Oncol Biol Phys. 1975 Oct-Nov;1(1-2):41–52. doi: 10.1016/0360-3016(75)90008-5. [DOI] [PubMed] [Google Scholar]
- Withers H. R. Response of tissues to multiple small dose fractions. Radiat Res. 1977 Jul;71(1):24–33. [PubMed] [Google Scholar]


