Abstract
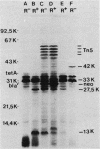
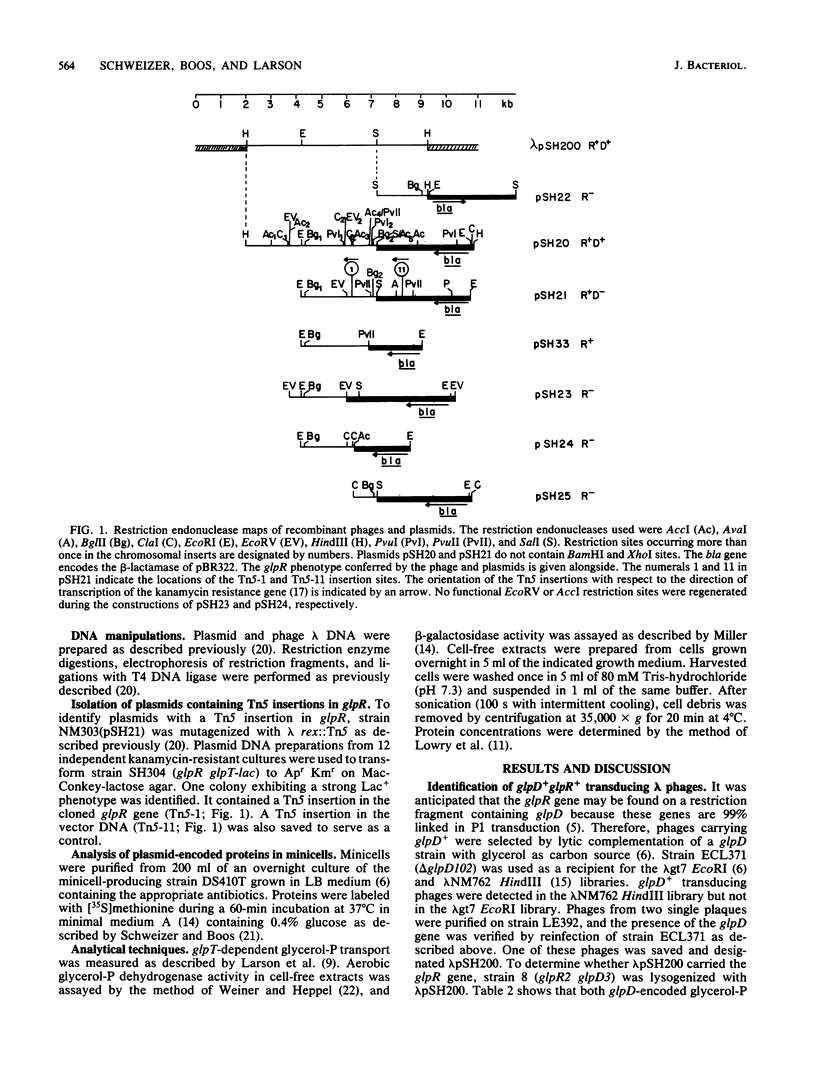
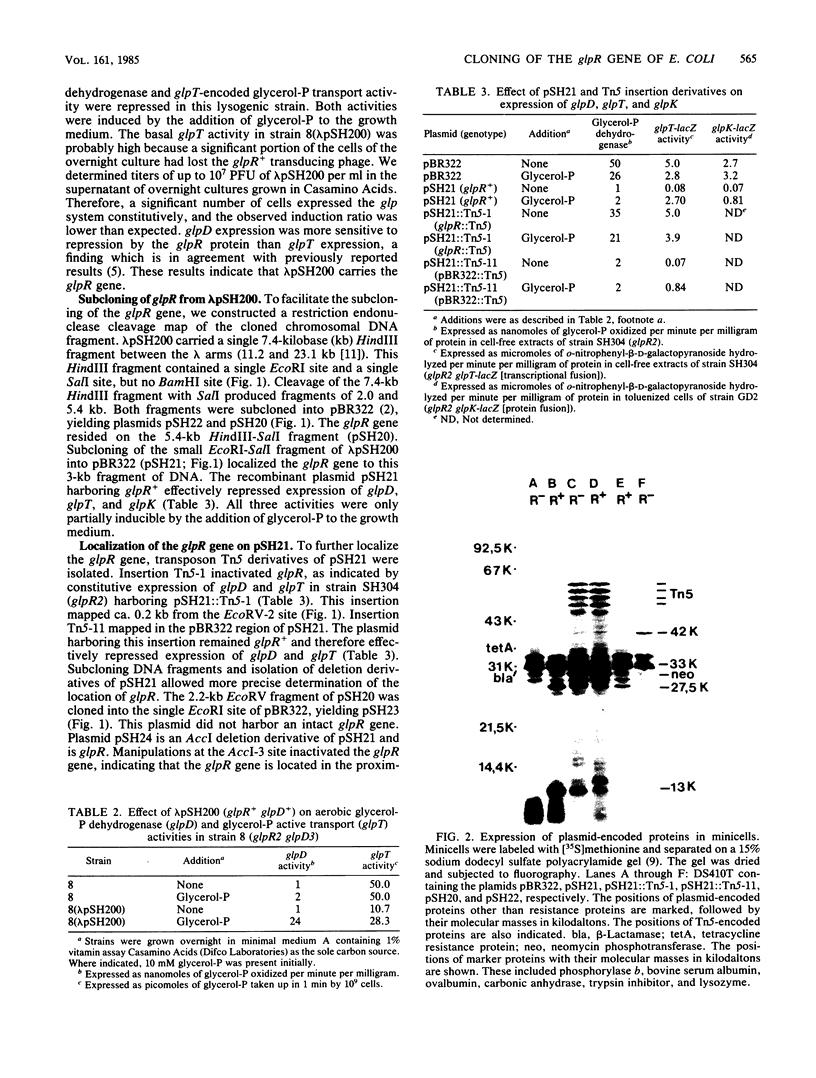
The glpR gene encoding the repressor for the glp regulon of Escherichia coli was cloned from a library of HindIII DNA fragments established in bacteriophage lambda. Phages harboring glpR were isolated by selection for sn-glycerol-3-phosphate dehydrogenase function encoded by glpD, which is adjacent to glpR on the E. coli linkage map. Restriction endonuclease analysis and recloning of DNA fragments localized glpR to a 3-kilobase-pair EcoRI-SalI segment of DNA. Strains exhibiting constitutive expression of the glp operons were strongly repressed after introduction of multicopy plasmids containing the glpR gene. Analysis of proteins labeled in minicells harboring either glpR+ recombinant plasmids or a glpR::Tn5 derivative showed that the glpR gene product is a protein with an apparent molecular weight of 33,000.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bremer E., Silhavy T. J., Weisemann J. M., Weinstock G. M. Lambda placMu: a transposable derivative of bacteriophage lambda for creating lacZ protein fusions in a single step. J Bacteriol. 1984 Jun;158(3):1084–1093. doi: 10.1128/jb.158.3.1084-1093.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C. A., Stearns G. W., 3rd, Prater W. E., Rheiner J. A., Johnson J. R. Characterization of a glpK transducing phage. Mol Gen Genet. 1984;193(2):376–378. doi: 10.1007/BF00330696. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Freedberg W. B., Lin E. C. Genetic control of L-alpha-glycerophosphate system in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):371–387. doi: 10.1016/0022-2836(68)90415-4. [DOI] [PubMed] [Google Scholar]
- Kuritzkes D. R., Zhang X. Y., Lin E. C. Use of phi(glp-lac) in studies of respiratory regulation of the Escherichia coli anaerobic sn-glycerol-3-phosphate dehydrogenase genes (glpAB). J Bacteriol. 1984 Feb;157(2):591–598. doi: 10.1128/jb.157.2.591-598.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larson T. J., Ehrmann M., Boos W. Periplasmic glycerophosphodiester phosphodiesterase of Escherichia coli, a new enzyme of the glp regulon. J Biol Chem. 1983 May 10;258(9):5428–5432. [PubMed] [Google Scholar]
- Larson T. J., Schumacher G., Boos W. Identification of the glpT-encoded sn-glycerol-3-phosphate permease of Escherichia coli, an oligomeric integral membrane protein. J Bacteriol. 1982 Dec;152(3):1008–1021. doi: 10.1128/jb.152.3.1008-1021.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E. C. Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol. 1976;30:535–578. doi: 10.1146/annurev.mi.30.100176.002535. [DOI] [PubMed] [Google Scholar]
- Ludtke D., Larson T. J., Beck C., Boos W. Only one gene is required for the glpT-dependent transport of sn-glycerol-3-phosphate in Escherichia coli. Mol Gen Genet. 1982;186(4):540–547. doi: 10.1007/BF00337962. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Richey D. P., Lin E. C. Importance of facilitated diffusion for effective utilization of glycerol by Escherichia coli. J Bacteriol. 1972 Nov;112(2):784–790. doi: 10.1128/jb.112.2.784-790.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein S. J., Jorgensen R. A., Postle K., Reznikoff W. S. The inverted repeats of Tn5 are functionally different. Cell. 1980 Mar;19(3):795–805. doi: 10.1016/s0092-8674(80)80055-9. [DOI] [PubMed] [Google Scholar]
- Schryvers A., Weiner J. H. The anaerobic sn-glycerol-3-phosphate dehydrogenase: cloning and expression of the glpA gene of Escherichia coli and identification of the glpA products. Can J Biochem. 1982 Mar;60(3):224–231. doi: 10.1139/o82-027. [DOI] [PubMed] [Google Scholar]
- Schweizer H., Boos W. Characterization of the ugp region containing the genes for the phoB dependent sn-glycerol-3-phosphate transport system of Escherichia coli. Mol Gen Genet. 1984;197(1):161–168. doi: 10.1007/BF00327937. [DOI] [PubMed] [Google Scholar]
- Schweizer H., Boos W. Cloning of the ugp region containing the structural genes for the pho regulon-dependent sn-glycerol-3-phosphate transport system of Escherichia coli. Mol Gen Genet. 1983;192(1-2):177–186. doi: 10.1007/BF00327664. [DOI] [PubMed] [Google Scholar]
- Shultz J., Silhavy T. J., Berman M. L., Fiil N., Emr S. D. A previously unidentified gene in the spc operon of Escherichia coli K12 specifies a component of the protein export machinery. Cell. 1982 Nov;31(1):227–235. doi: 10.1016/0092-8674(82)90422-6. [DOI] [PubMed] [Google Scholar]
- Weiner J. H., Heppel L. A. Purification of the membrane-bound and pyridine nucleotide-independent L-glycerol 3-phosphate dehydrogenase from Escherichia coli. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1360–1365. doi: 10.1016/0006-291x(72)90222-7. [DOI] [PubMed] [Google Scholar]