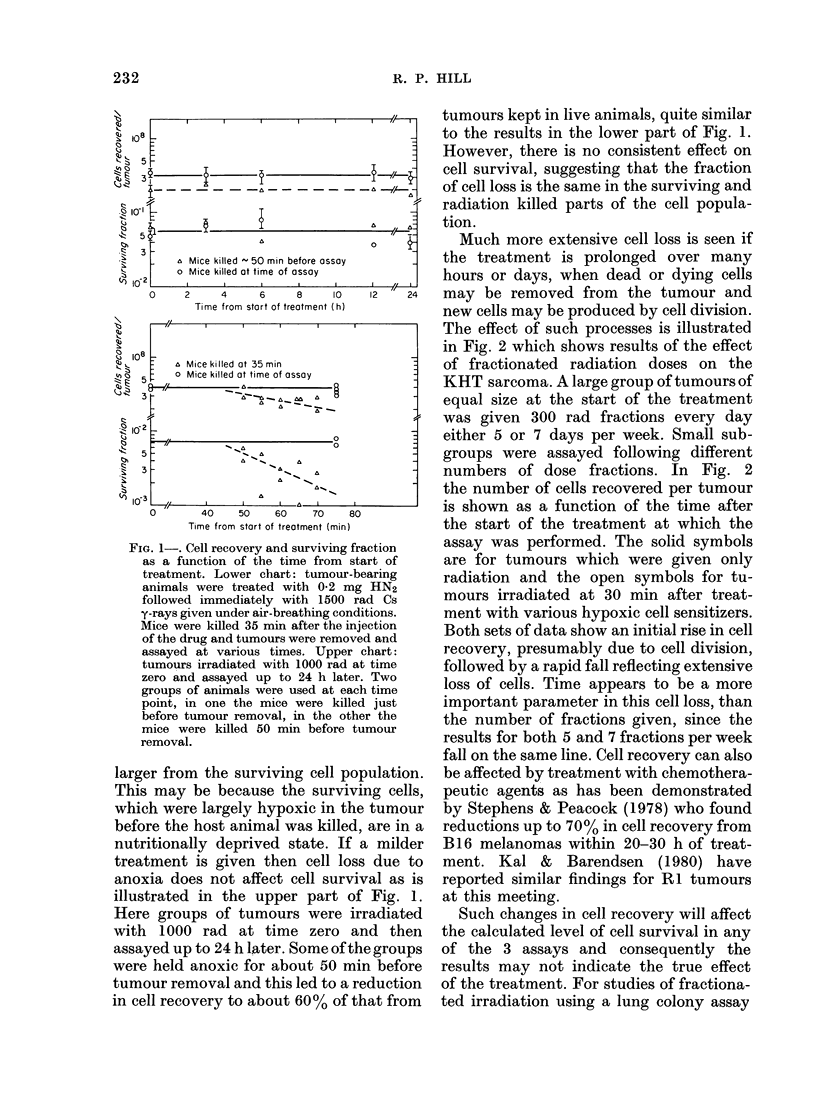
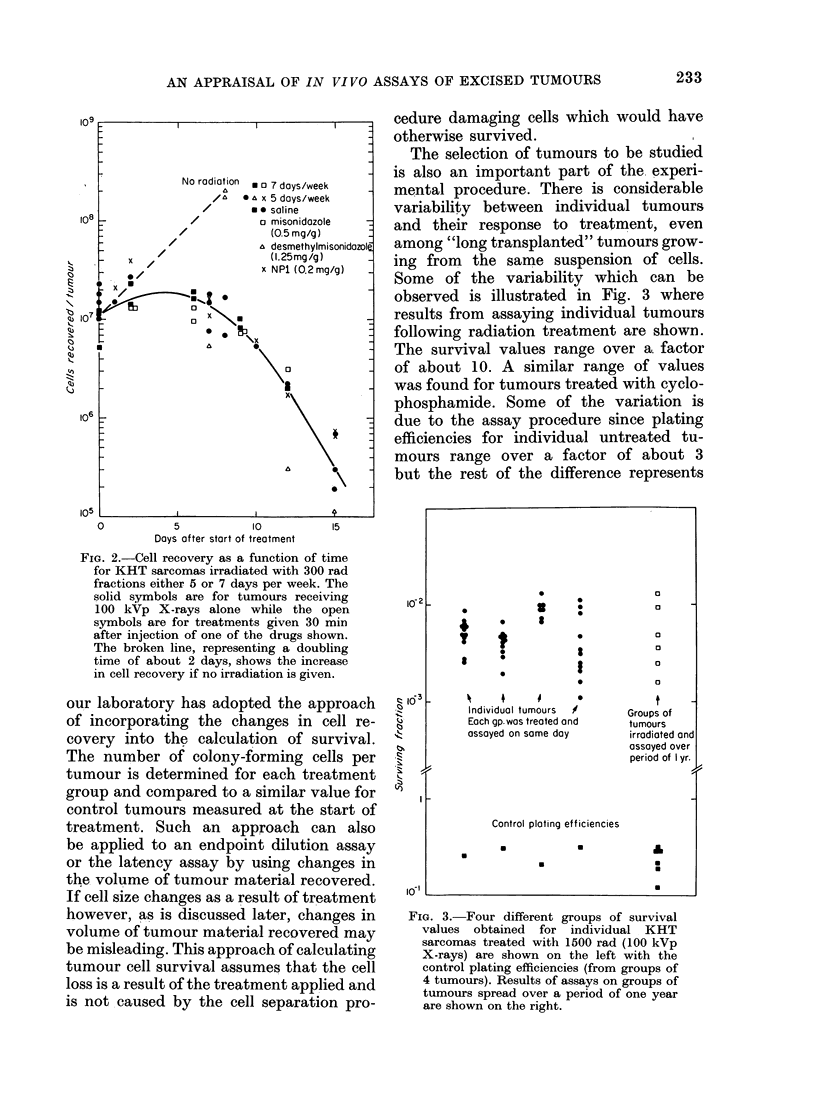
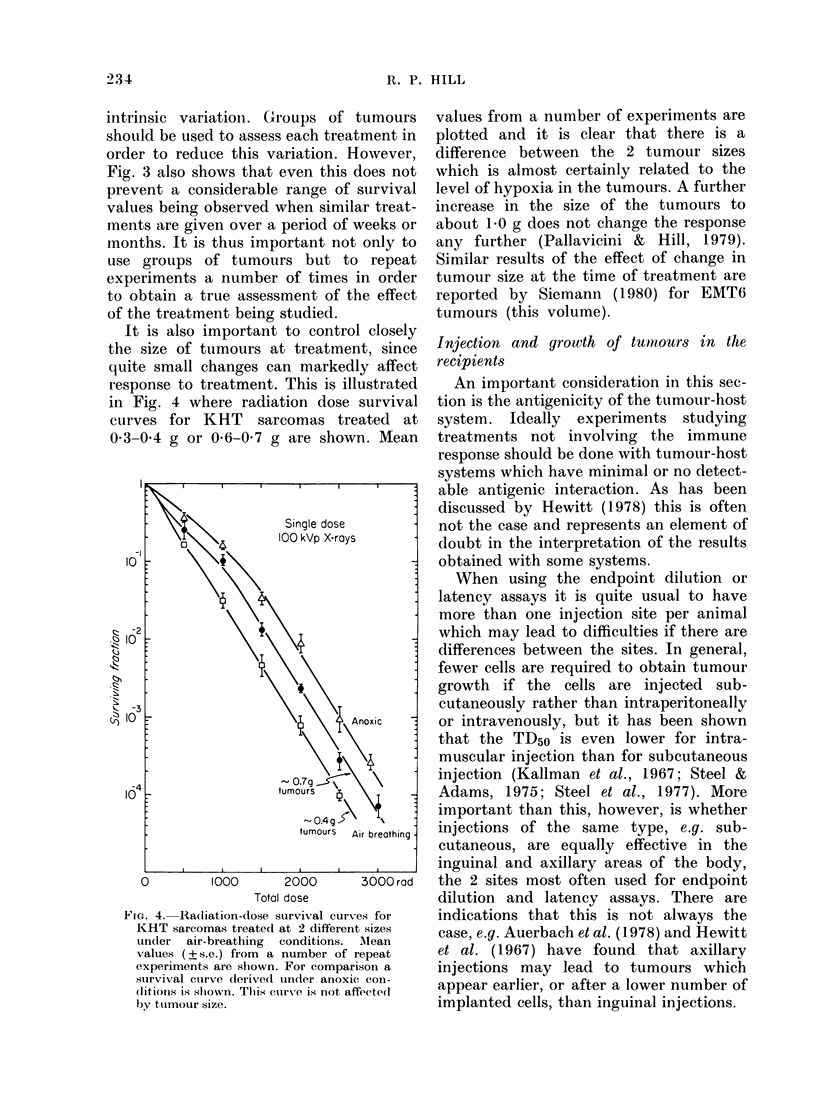
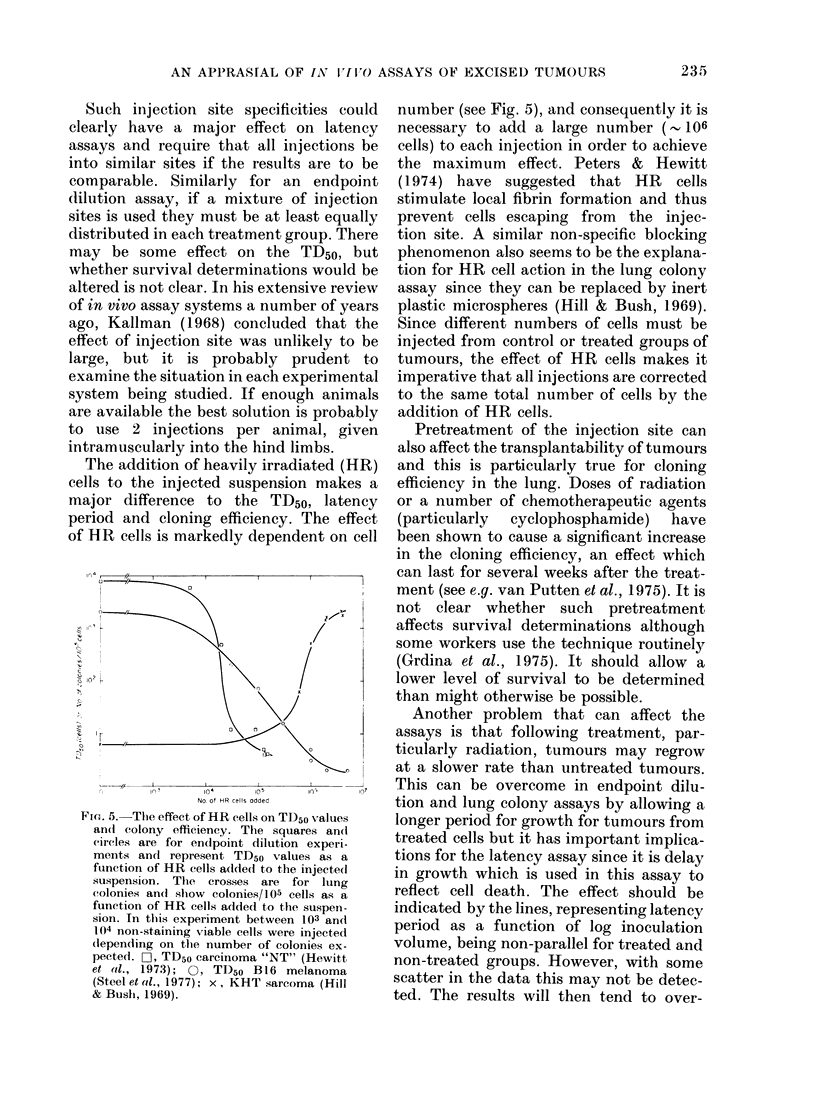
Abstract
Three in vivo assays of excised tumours are compared, the endpoint dilution assay, the tumour latency assay and the lung colony assay. The assay procedures are discussed in 2 phases; the preparation of the required cell suspension and the injection and growth of the tumour cells in recipients. Factors reviewed include those affecting recovery of cells during the suspension procedure, the variability of tumour response, the importance of the site of injection, the effect of heavily irradiated cells and non-lethal effects of radiation. Specific aspects of the lung colony assays are also described. Results of an experiment to compare the 3 assay procedures with that of an in vitro agar colony assay are presented and indicate reasonable agreement for the KHT sarcoma except perhaps for the latency assay at low levels of survival. A list of recommendations of ways to minimize some of the potential problems and a comparison of assay procedures is presented.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach R., Morrissey L. W., Sidky Y. A. Regional differences in the incidence and growth of mouse tumors following intradermal or subcutaneous inoculation. Cancer Res. 1978 Jun;38(6):1739–1744. [PubMed] [Google Scholar]
- CLIFTON K. H., DRAPER N. R. SURVIVAL-CURVES OF SOLID TRANSPLANTABLE TUMOUR CELLS IRRADIATED IN VIVO: A METHOD OF DETERMINATION AND STATISTICAL EVALUATION; COMPARISON OF CELL-SURVIVAL AND 32-P-UPTAKE INTO DNA. Int J Radiat Biol Relat Stud Phys Chem Med. 1963 Dec;7:515–535. doi: 10.1080/09553006314551561. [DOI] [PubMed] [Google Scholar]
- Douple E. B., Richmond R. C. Radiosensitization of hypoxic tumor cells by cis- and trans-dichlorodiammineplatinum (II). Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1369–1372. doi: 10.1016/0360-3016(79)90672-2. [DOI] [PubMed] [Google Scholar]
- Grdina D. J., Basic I., Mason K. A., Withers H. R. Radiation response of clonogenic cell populations separated from fibrosarcoma. Radiat Res. 1975 Sep;63(3):483–493. [PubMed] [Google Scholar]
- Grdina D. J., Hittelman W. N., White R. A., Meistrich M. L. Relevance of density, size and DNA content of tumour cells to the lung colony assay. Br J Cancer. 1977 Dec;36(6):659–669. doi: 10.1038/bjc.1977.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEWITT H. B., WILSON C. W. A survival curve for mammalian leukaemia cells irradiated in vivo (implications for the treatment of mouse leukaemia by whole-body irradiation). Br J Cancer. 1959 Mar;13(1):69–75. doi: 10.1038/bjc.1959.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt H. B., Chan D. P., Blake E. R. Survival curves for clonogenic cells of a murine keratinizing squamous carcinoma irradiated in vivo or under hypoxic conditions. Int J Radiat Biol Relat Stud Phys Chem Med. 1967;12(6):535–549. doi: 10.1080/09553006714551151. [DOI] [PubMed] [Google Scholar]
- Hewitt H. B. The choice of animal tumors for experimental studies of cancer therapy. Adv Cancer Res. 1978;27:149–200. doi: 10.1016/s0065-230x(08)60932-x. [DOI] [PubMed] [Google Scholar]
- Hill R. P., Bush R. S. A lung-colony assay to determine the radiosensitivity of cells of a solid tumour. Int J Radiat Biol Relat Stud Phys Chem Med. 1969 Jul;15(5):435–444. doi: 10.1080/09553006914550721. [DOI] [PubMed] [Google Scholar]
- Jirtle R., Clifton K. H. The effect of tumor size and host anemia on tumor cell survival after irradiation. Int J Radiat Oncol Biol Phys. 1978 May-Jun;4(5-6):395–400. doi: 10.1016/0360-3016(78)90068-8. [DOI] [PubMed] [Google Scholar]
- Kal H. B., Barendsen G. W. Cell survival and growth delay in rat R-1 tumours after radiation and vinblastine treatment. Br J Cancer Suppl. 1980 Apr;4:275–278. [PMC free article] [PubMed] [Google Scholar]
- Kallman R. F., Silini G., Van Putten L. M. Factors influencing the quantitative estimation of the in vivo survival of cells from solid tumors. J Natl Cancer Inst. 1967 Sep;39(3):539–549. [PubMed] [Google Scholar]
- PORTER E. H., BERRY R. J. THE EFFICIENT DESIGN OF TRANSPLANTABLE TUMOUR ASSAYS. Br J Cancer. 1963 Dec;17:583–595. doi: 10.1038/bjc.1963.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallavicini M. G., Hill R. P. Relationship of tumor blood flow with radiation and drug response. Int J Radiat Oncol Biol Phys. 1979 Oct;5(10):1767–1772. doi: 10.1016/0360-3016(79)90559-5. [DOI] [PubMed] [Google Scholar]
- Peters L. J., Hewitt H. B. The influence of fibrin formation on the transplantability of murine tumour cells: implications for the mechanism of the Révész effect. Br J Cancer. 1974 Apr;29(4):279–291. doi: 10.1038/bjc.1974.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice L., Urano M., Suit H. D. The radiosensitivity of a murine fibrosarcoma as measured by three cell survival assays. Br J Cancer Suppl. 1980 Apr;4:240–244. [PMC free article] [PubMed] [Google Scholar]
- Siemann D. W. Tumour size: a factor influencing the isoeffect analysis of tumour response to combined modalities. Br J Cancer Suppl. 1980 Apr;4:294–298. [PMC free article] [PubMed] [Google Scholar]
- Steel G. G., Adams K. Stem-cell survival and tumor control in the Lewis lung carcinoma. Cancer Res. 1975 Jun;35(6):1530–1535. [PubMed] [Google Scholar]
- Steel G. G., Adams K., Stephens T. C. Clonogenic assays in the B16 melanoma: response to cyclophosphamide. Br J Cancer. 1977 Nov;36(5):618–624. doi: 10.1038/bjc.1977.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens T. C., Peacock J. H. Cell yield and cell survival following chemotherapy of the B16 melanoma. Br J Cancer. 1978 Nov;38(5):591–598. doi: 10.1038/bjc.1978.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. C. The colony forming efficiency of single cells and cell aggregates from a spontaneous mouse mammary tumour using the lung colony assay. Br J Cancer. 1974 Oct;30(4):332–336. doi: 10.1038/bjc.1974.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J. E., Rauth A. M. An in vitro assay to measure the viability of KHT tumor cells not previously exposed to culture conditions. Radiat Res. 1974 May;58(2):262–276. [PubMed] [Google Scholar]
- van Putten L. M., Kram L. K., van Dierendonck H. H., Smink T., Füzy M. Enhancement by drugs of metastatic lung nodule formation after intravenous tumour cell injection. Int J Cancer. 1975 Apr 15;15(4):588–595. doi: 10.1002/ijc.2910150408. [DOI] [PubMed] [Google Scholar]


