Abstract
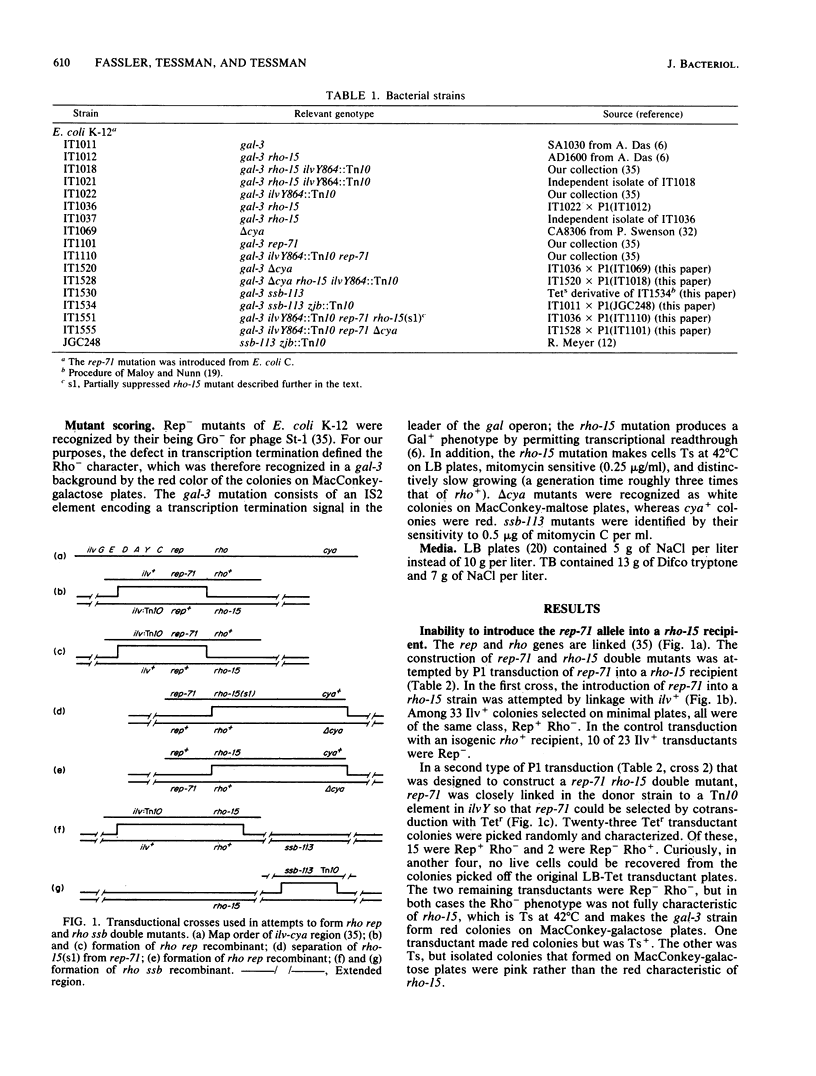
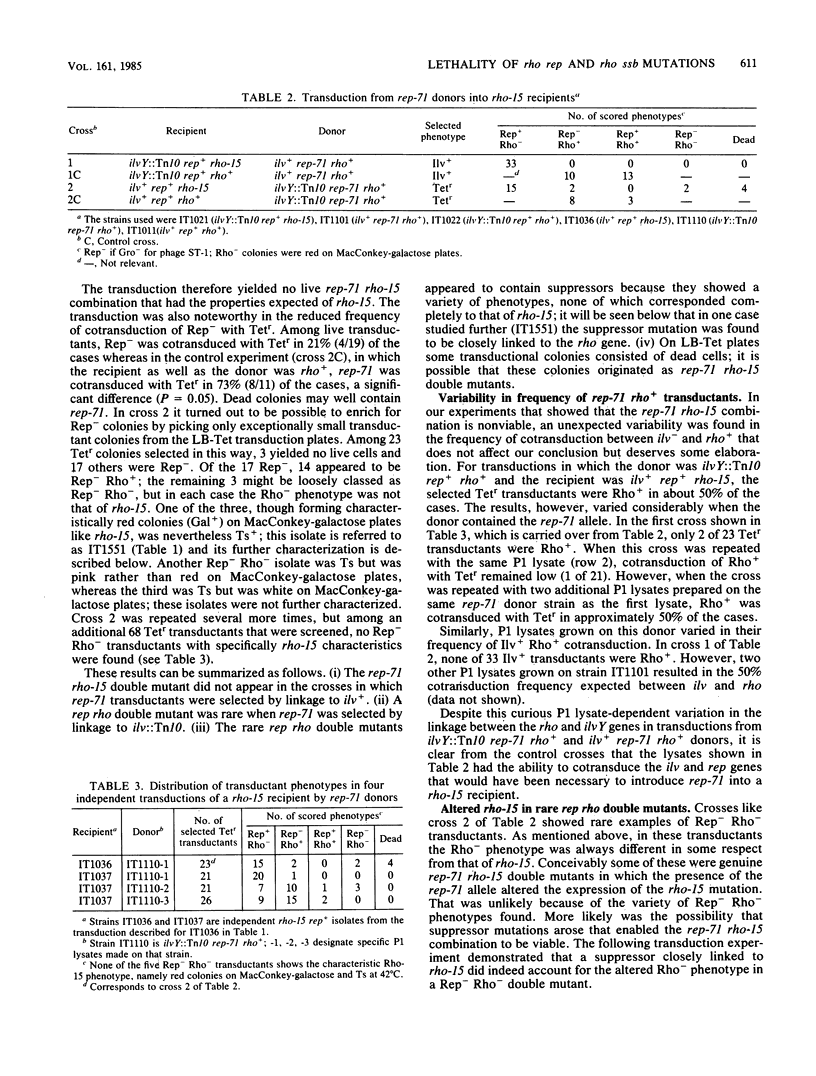
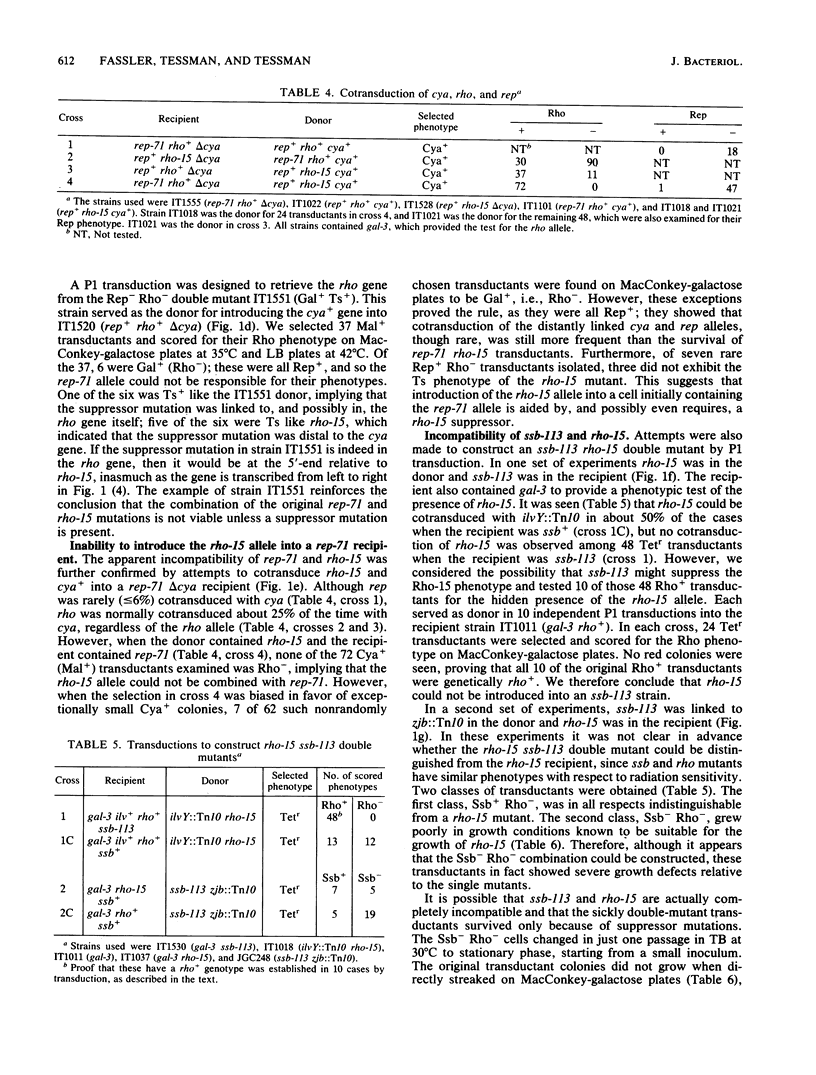
The similarity of rho mutants to rep and ssb mutants in sensitivity to UV light and in recombination deficiency suggested that the function of the Rho protein might be related to that of Rep and Ssb. In support of that idea, we found that rho rep and rho ssb double mutants are either nonviable, or at best only marginally viable. Viability could be restored by suppressor mutations, one of which mapped either in the rho gene or close to its 5'-end. Rho may thus share a role with Rep and Ssb in replication and the structural maintenance of DNA; a multifunctional Rho protein could account for the diversity of the defects seen in rho mutants, some of which appear to have no relation to the defect in transcription termination.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumberg S., Lovett M. G. Reduced recovery of plasmid transconjugants in crosses with Escherichia coli rho mutant recipients. Plasmid. 1977 Nov;1(1):118–122. doi: 10.1016/0147-619x(77)90014-2. [DOI] [PubMed] [Google Scholar]
- Beckmann J. S., Tichauer Y., Daniel V., Littauer U. Z. Binding of the termination factor rho to DNA. Biochem Biophys Res Commun. 1971 May 21;43(4):806–813. doi: 10.1016/0006-291x(71)90688-7. [DOI] [PubMed] [Google Scholar]
- Blumenthal R. M., Reeh S., Pedersen S. Regulation of transcription factor rho and the alpha subunit of RNA polymerase in Escherichia coli B/r. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2285–2288. doi: 10.1073/pnas.73.7.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S., Albrechtsen B., Pedersen S., Klemm P. Localization and regulation of the structural gene for transcription-termination factor rho of Escherichia coli. J Mol Biol. 1982 Dec 5;162(2):283–298. doi: 10.1016/0022-2836(82)90527-7. [DOI] [PubMed] [Google Scholar]
- Calendar R., Lindqvist B., Sironi G., Clark A. J. Characterization of REP- mutants and their interaction with P2 phage. Virology. 1970 Jan;40(1):72–83. doi: 10.1016/0042-6822(70)90380-6. [DOI] [PubMed] [Google Scholar]
- Das A., Court D., Adhya S. Isolation and characterization of conditional lethal mutants of Escherichia coli defective in transcription termination factor rho. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1959–1963. doi: 10.1073/pnas.73.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Merril C., Adhya S. Interaction of RNA polymerase and rho in transcription termination: coupled ATPase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4828–4832. doi: 10.1073/pnas.75.10.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T., Dressler D. H., Hathaway A. THE ABORTIVE REPLICATION OF PhiX174 DNA IN A RECOMBINATION-DEFICIENT MUTANT OF Escherichia coli. Proc Natl Acad Sci U S A. 1967 Mar;57(3):813–820. doi: 10.1073/pnas.57.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T., Iwaya M., Larison L. L. The rep mutation. II. Its effect on Escherichia coli and on the replication of bacteriophage phi X174. Virology. 1972 Aug;49(2):486–496. doi: 10.1016/0042-6822(72)90500-4. [DOI] [PubMed] [Google Scholar]
- Fassler J. S., Tessman I. Relation between UV suppression of polarity in phi X174 and UV sensitivity of rho mutants. J Virol. 1981 Mar;37(3):955–962. doi: 10.1128/jvi.37.3.955-962.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassberg J., Meyer R. R., Kornberg A. Mutant single-strand binding protein of Escherichia coli: genetic and physiological characterization. J Bacteriol. 1979 Oct;140(1):14–19. doi: 10.1128/jb.140.1.14-19.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. R., Hurwitz J. Studies on termination of in vitro ribonucleic acid synthesis by rho factor. J Biol Chem. 1972 Sep 10;247(17):5637–5645. [PubMed] [Google Scholar]
- Guterman S. K., Howitt C. L. Rifampicin supersensitivity of rho strains of E. coli, and suppression by sur mutation. Mol Gen Genet. 1979 Jan 16;169(1):27–34. doi: 10.1007/BF00267541. [DOI] [PubMed] [Google Scholar]
- Housley P. R., Whitfield H. J. Transcription termination factor rho from wild type and rho-111 strains of Salmonella typhimurium. J Biol Chem. 1982 Mar 10;257(5):2569–2577. [PubMed] [Google Scholar]
- Imai M., Shigesada K. Studies on the altered rho factor in a nitA mutants of Escherichia coli defective in transcription termination. I. Characterization and quantitative determination of rho in cell extracts. J Mol Biol. 1978 Apr 25;120(4):451–466. doi: 10.1016/0022-2836(78)90348-0. [DOI] [PubMed] [Google Scholar]
- Inoko H., Shigesada K., Imai M. Isolation and characterization of conditional-lethal rho mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1162–1166. doi: 10.1073/pnas.74.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A., Scott J. F., Bertsch L. L. ATP utilization by rep protein in the catalytic separation of DNA strands at a replicating fork. J Biol Chem. 1978 May 10;253(9):3298–3304. [PubMed] [Google Scholar]
- Lowery-Goldhammer C., Richardson J. P. An RNA-dependent nucleoside triphosphate phosphohydrolase (ATPase) associated with rho termination factor. Proc Natl Acad Sci U S A. 1974 May;71(5):2003–2007. doi: 10.1073/pnas.71.5.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T., Takanami M. Observations on the structure of the termination factor rho and its attachment to DNA. J Mol Biol. 1972 Nov 28;71(3):799–802. doi: 10.1016/s0022-2836(72)80041-x. [DOI] [PubMed] [Google Scholar]
- Richardson J. P. Activation of rho protein ATPase requires simultaneous interaction at two kinds of nucleic acid-binding sites. J Biol Chem. 1982 May 25;257(10):5760–5766. [PubMed] [Google Scholar]
- Richardson J. P., Grimley C., Lowery C. Transcription termination factor rho activity is altered in Escherichia coli with suA gene mutations. Proc Natl Acad Sci U S A. 1975 May;72(5):1725–1728. doi: 10.1073/pnas.72.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Scott J. F., Eisenberg S., Bertsch L. L., Kornberg A. A mechanism of duplex DNA replication revealed by enzymatic studies of phage phi X174: catalytic strand separation in advance of replication. Proc Natl Acad Sci U S A. 1977 Jan;74(1):193–197. doi: 10.1073/pnas.74.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E. C. Ultraviolet-sensitive mutator mutU4 of Escherichia coli inviable with polA. J Bacteriol. 1973 Jan;113(1):161–166. doi: 10.1128/jb.113.1.161-166.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal N., Delius H., Kornberg T., Gefter M. L., Alberts B. A DNA-unwinding protein isolated from Escherichia coli: its interaction with DNA and with DNA polymerases. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3537–3541. doi: 10.1073/pnas.69.12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L. D., Gottesman M., Tomczak K., Gottesman S. Hyperdegradation of proteins in Escherichia coli rho mutants. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1623–1627. doi: 10.1073/pnas.76.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov G. B., Filkova E. V., Skavronskaya A. G., Saenko A. S., Sinzinis B. I. Loss and restoration of viability of E. coli due to combinations of mutations affecting DNA polymerase I and repair activities. Mol Gen Genet. 1973 Mar 1;121(2):139–150. doi: 10.1007/BF00277528. [DOI] [PubMed] [Google Scholar]
- Swenson P. A., Joshi J. G., Schenley R. L. Regulation of cessation of respiration and killing by cyclic 3',5'-adenosine monophosphate and its receptor protein after far-ultraviolet irradiation of Escherichia coli. Mol Gen Genet. 1978 Feb 16;159(2):125–130. doi: 10.1007/BF00270885. [DOI] [PubMed] [Google Scholar]
- Swenson P. A., Joshi J. G., Schenley R. L. Regulation of cessation of respiration and killing by cyclic 3',5'-adenosine monophosphate and its receptor protein after far-ultraviolet irradiation of Escherichia coli. Mol Gen Genet. 1978 Feb 16;159(2):125–130. doi: 10.1007/BF00270885. [DOI] [PubMed] [Google Scholar]
- Tessman E. S., Peterson P. K. Suppression of the ssb-1 and ssb-113 mutations of Escherichia coli by a wild-type rep gene, NaCl, and glucose. J Bacteriol. 1982 Nov;152(2):572–583. doi: 10.1128/jb.152.2.572-583.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman I., Fassler J. S., Bennett D. C. Relative map location of the rep and rho genes of Escherichia coli. J Bacteriol. 1982 Sep;151(3):1637–1640. doi: 10.1128/jb.151.3.1637-1640.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieg J., Maples V. F., Kushner S. R. Recombinant levels of Escherichia coli K-12 mutants deficient in various replication, recombination, or repair genes. J Bacteriol. 1978 Jun;134(3):958–966. doi: 10.1128/jb.134.3.958-966.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


