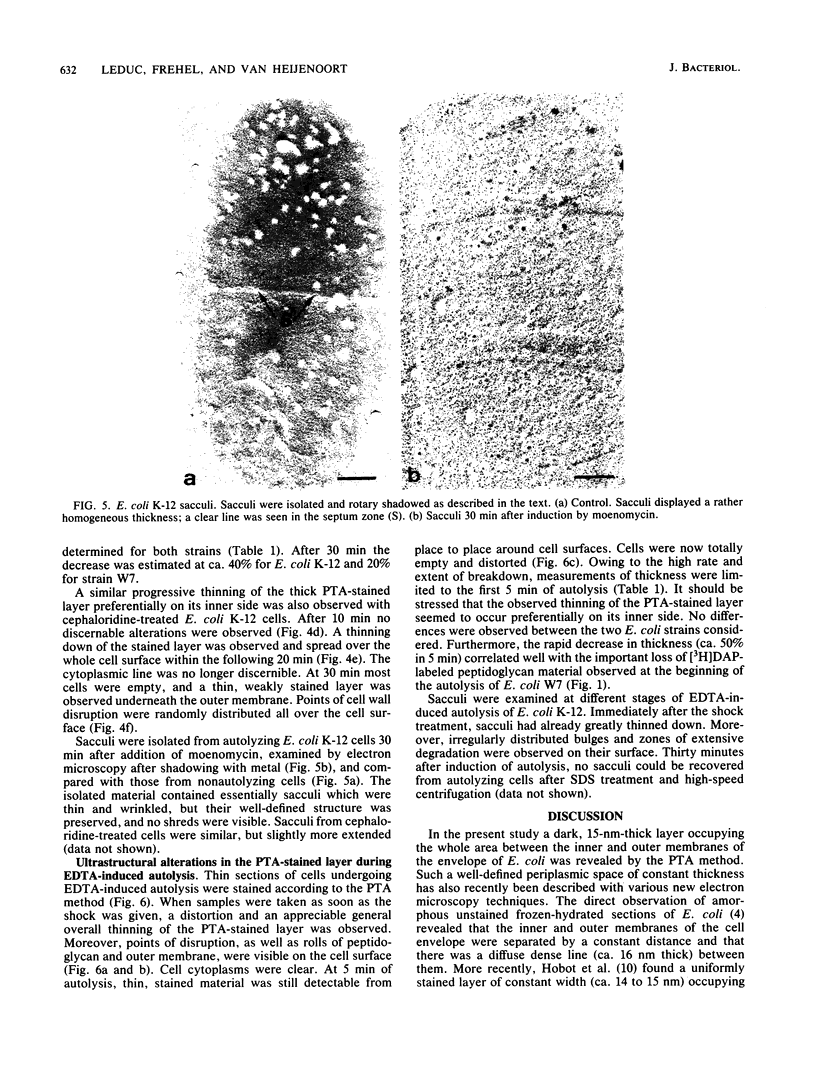
Abstract
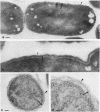
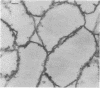
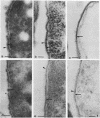
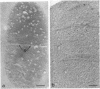
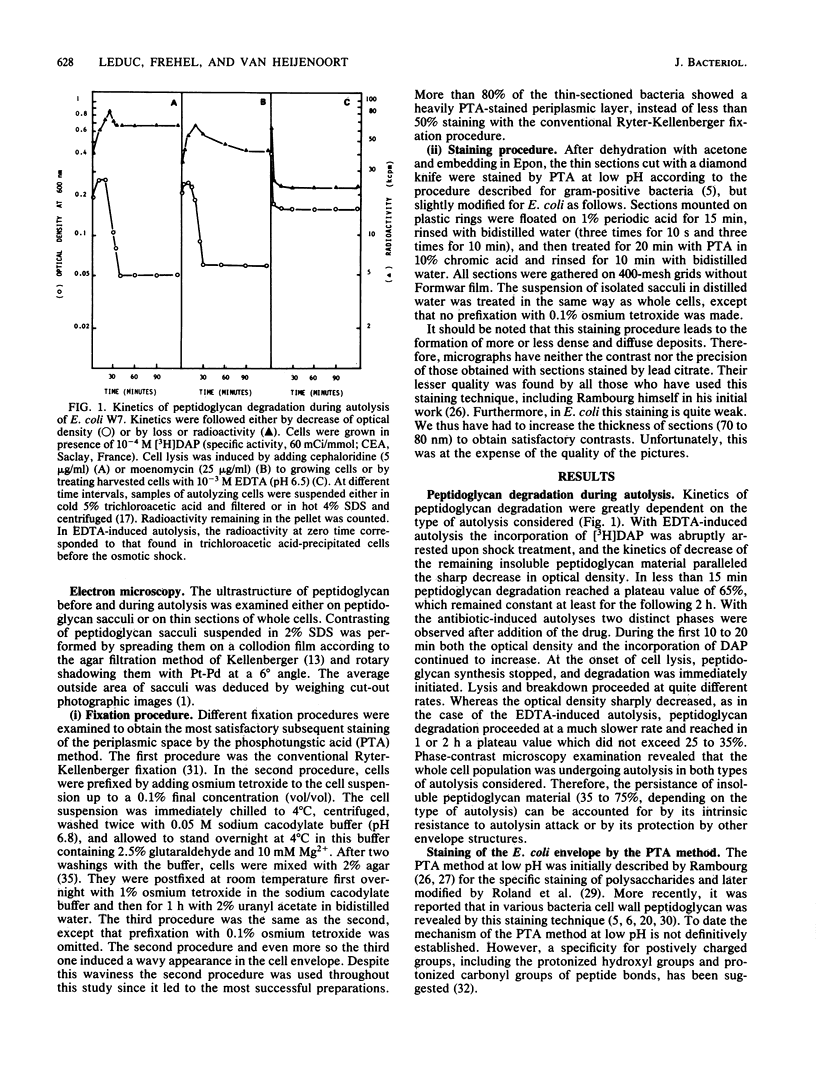
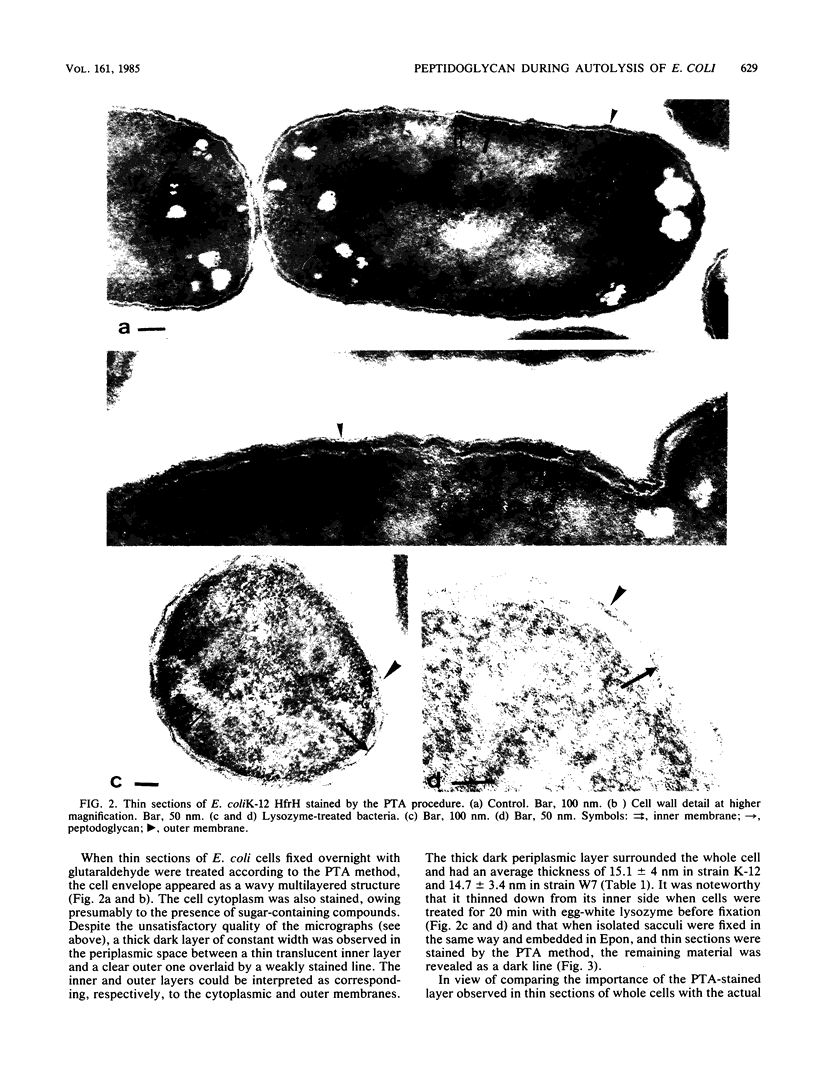
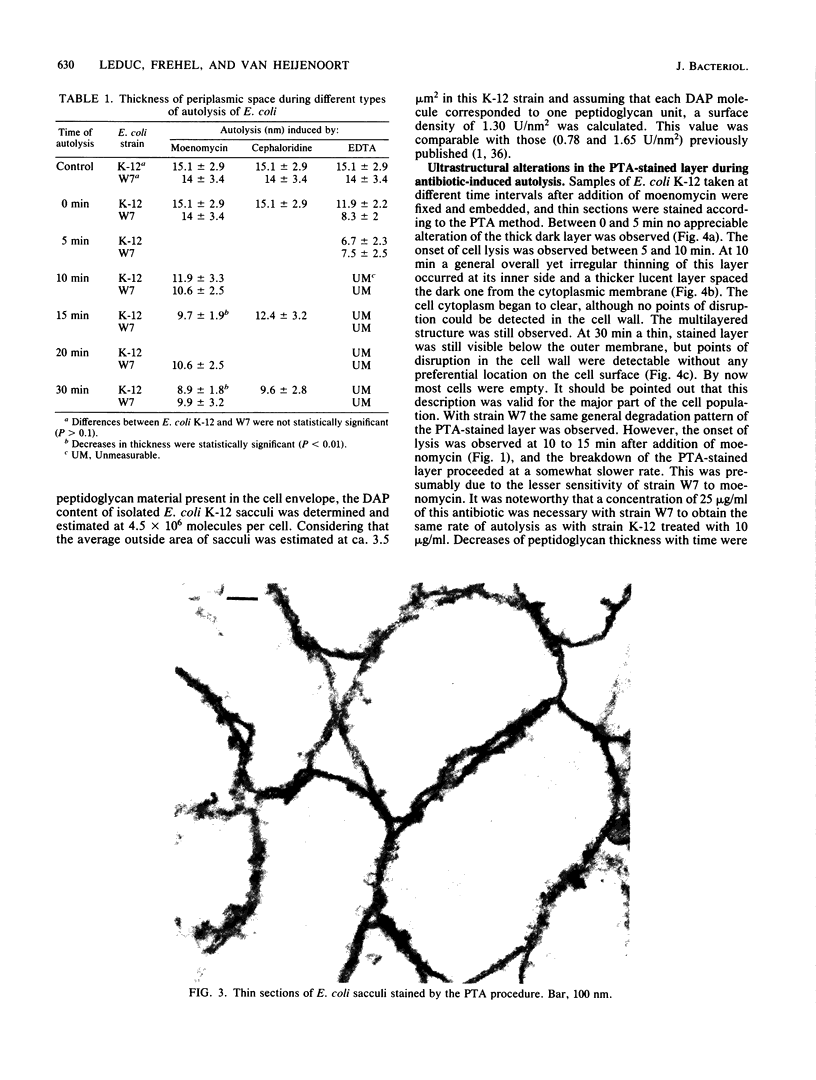
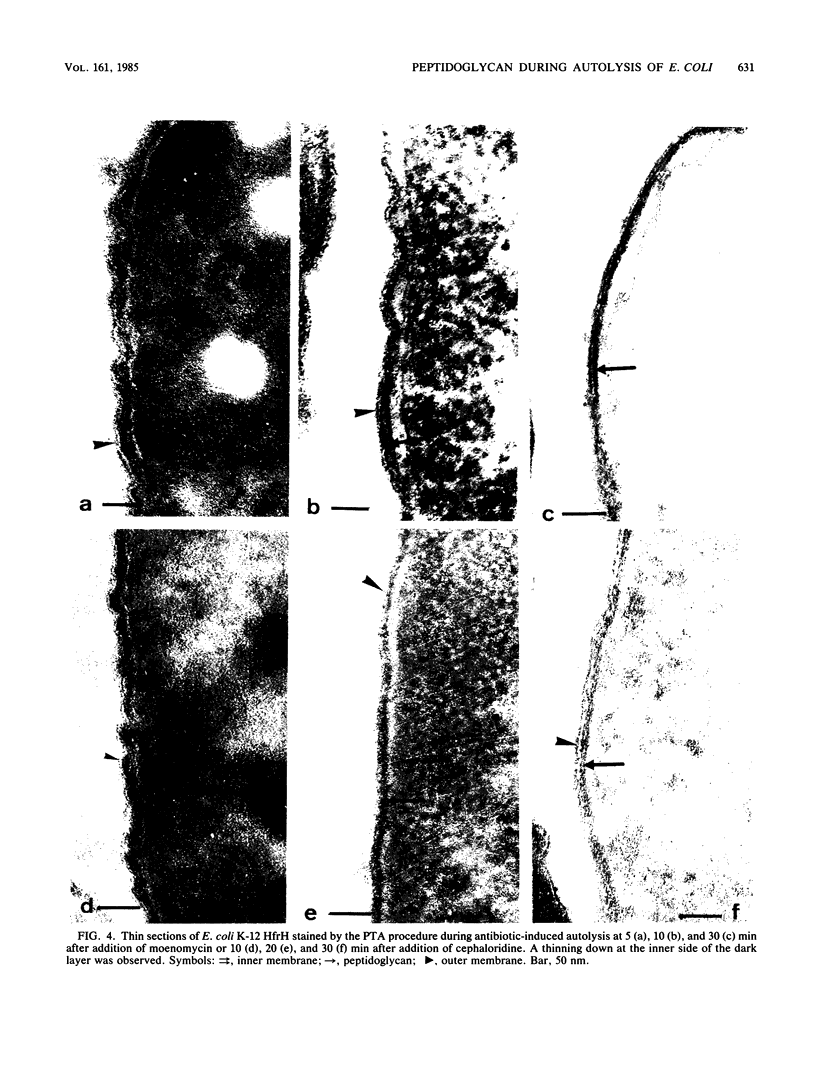
The kinetics of peptidoglycan degradation were examined under different conditions of autolysis of Escherichia coli. With cephaloridine- or moenomycin-induced autolysis, degradation did not exceed 25 to 35%, whereas in EDTA-induced autolysis it rapidly reached 65 to 70%. When nonautolyzing cells were fixed overnight with glutaraldehyde, followed by an osmium fixation, and thin sections were stained by the phosphotungstic acid method, a dark, 15-nm-thick layer of uniform appearance and constant width occupied the whole area between the inner and outer membranes of the envelope. The stained material was tentatively identified with peptidoglycan. Ultrastructural changes in this phosphotungstic acid-stained periplasmic space were investigated at different time intervals after induction of autolysis. In all cases, breakdown proceeded over the whole cell surface. During antibiotic-induced autolysis a progressive thinning down limited to the inner side of the layer was observed. During EDTA-induced autolysis, the rapid decrease in thickness correlated well with the important loss of material labeled with [3H]diaminopimelic acid. Considering these changes and the insufficient amounts of peptidoglycan (1.3 U/nm2) necessary to account for a regularly structured polymer occupying the whole 15-nm layer, it was speculated that peptidoglycan might be unevenly distributed throughout the periplasmic space.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V., Gnirke H., Henning U., Rehn K. Model for the structure of the shape-maintaining layer of the Escherichia coli cell envelope. J Bacteriol. 1973 Jun;114(3):1264–1270. doi: 10.1128/jb.114.3.1264-1270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G., Reichler J., Park J. T. Evidence for multisite growth of Escherichia coli murein involving concomitant endopeptidase and transpeptidase activities. J Bacteriol. 1983 Oct;156(1):386–392. doi: 10.1128/jb.156.1.386-392.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- Dubochet J., McDowall A. W., Menge B., Schmid E. N., Lickfeld K. G. Electron microscopy of frozen-hydrated bacteria. J Bacteriol. 1983 Jul;155(1):381–390. doi: 10.1128/jb.155.1.381-390.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehel C., Robbe P., Tinelli R., Ryter A. Relationship between biochemical and cytochemical results obtained on Bacillus megaterium and Bacillus subtilis cell-wall polysaccharides. J Ultrastruct Res. 1982 Oct;81(1):78–87. doi: 10.1016/s0022-5320(82)90042-9. [DOI] [PubMed] [Google Scholar]
- Frehel C., Ryter A. Electron microscopic cytochemical study of cell-wall polysaccharides in Bacillus subtilis and two strains of Bacillus megaterium. J Ultrastruct Res. 1982 Oct;81(1):66–77. doi: 10.1016/s0022-5320(82)90041-7. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Essig P., Martin H. H. Characterization of minor fragments after digestion of Escherichia coli murein with endo-N,O-diacetylmuramidase from Chalaropsis, and determination of glycan chain length. FEBS Lett. 1982 Feb 8;138(1):109–112. doi: 10.1016/0014-5793(82)80406-7. [DOI] [PubMed] [Google Scholar]
- Goodell E. W., Schwarz U. Enzymes synthesizing and hydrolyzing murein in Escherichia coli. Topographical distribution over the cell envelope. Eur J Biochem. 1977 Nov 15;81(1):205–210. doi: 10.1111/j.1432-1033.1977.tb11942.x. [DOI] [PubMed] [Google Scholar]
- Hakenbeck R., Goodell E. W., Schwarz U. Compartmentalization of murein hydrolases in the envelope of Escherichia coli. FEBS Lett. 1974 Apr 1;40(2):261–264. doi: 10.1016/0014-5793(74)80240-1. [DOI] [PubMed] [Google Scholar]
- Hobot J. A., Carlemalm E., Villiger W., Kellenberger E. Periplasmic gel: new concept resulting from the reinvestigation of bacterial cell envelope ultrastructure by new methods. J Bacteriol. 1984 Oct;160(1):143–152. doi: 10.1128/jb.160.1.143-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K., Hirota Y., Schwarz U. Mutants of Escherichia coli defective in penicillin-insensitive murein DD-endopeptidase. Mol Gen Genet. 1983;189(2):215–221. doi: 10.1007/BF00337807. [DOI] [PubMed] [Google Scholar]
- Kitano K., Tomasz A. Triggering of autolytic cell wall degradation in Escherichia coli by beta-lactam antibiotics. Antimicrob Agents Chemother. 1979 Dec;16(6):838–848. doi: 10.1128/aac.16.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L., Burdett I. D. The variable T model for gram-negative morphology. J Gen Microbiol. 1984 Sep;130(9):2325–2338. doi: 10.1099/00221287-130-9-2325. [DOI] [PubMed] [Google Scholar]
- Leduc M., Kasra R., van Heijenoort J. Induction and control of the autolytic system of Escherichia coli. J Bacteriol. 1982 Oct;152(1):26–34. doi: 10.1128/jb.152.1.26-34.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc M., Rousseau M., van Heijenoort J. Structure of the cell wall of Bacillus species C.I.P. 76-111. Eur J Biochem. 1977 Oct 17;80(1):153–163. doi: 10.1111/j.1432-1033.1977.tb11867.x. [DOI] [PubMed] [Google Scholar]
- Leduc M., van Heijenoort J. Autolysis of Escherichia coli. J Bacteriol. 1980 Apr;142(1):52–59. doi: 10.1128/jb.142.1.52-59.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- Martin H. H. Bacterial protoplasts--a review. J Theor Biol. 1963 Jul;5(1):1–34. doi: 10.1016/0022-5193(63)90034-1. [DOI] [PubMed] [Google Scholar]
- Mengin-Lecreulx D., Flouret B., van Heijenoort J. Cytoplasmic steps of peptidoglycan synthesis in Escherichia coli. J Bacteriol. 1982 Sep;151(3):1109–1117. doi: 10.1128/jb.151.3.1109-1117.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldmixon E. H., Glauser S., Higgins M. L. Two proposed general configurations for bacterial cell wall peptidoglycans shown by space-filling molecular models. Biopolymers. 1974;13(10):2037–2060. doi: 10.1002/bip.1974.360131008. [DOI] [PubMed] [Google Scholar]
- Parquet C., Flouret B., Leduc M., Hirota Y., van Heijenoort J. N-acetylmuramoyl-L-alanine amidase of Escherichia coli K12. Possible physiological functions. Eur J Biochem. 1983 Jun 15;133(2):371–377. doi: 10.1111/j.1432-1033.1983.tb07472.x. [DOI] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Roland J. C., Lembi C. A., Morré D. J. Phosphotungstic acid-chromic acid as a selective electron-dense stain for plasma membranes of plant cells. Stain Technol. 1972 Jul;47(4):195–200. doi: 10.3109/10520297209116484. [DOI] [PubMed] [Google Scholar]
- Schulman H., Kennedy E. P. Localization of membrane-derived oligosaccharides in the outer envelope of Escherichia coli and their occurrence in other Gram-negative bacteria. J Bacteriol. 1979 Jan;137(1):686–688. doi: 10.1128/jb.137.1.686-688.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Woldringh C. L., Mirelman D. Constant peptidoglycan density in the sacculus of Escherichia coli B/r growing at different rates. FEBS Lett. 1979 Feb 1;98(1):29–32. doi: 10.1016/0014-5793(79)80144-1. [DOI] [PubMed] [Google Scholar]