Abstract
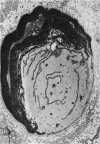
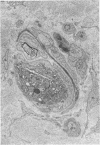
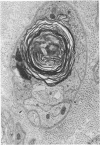
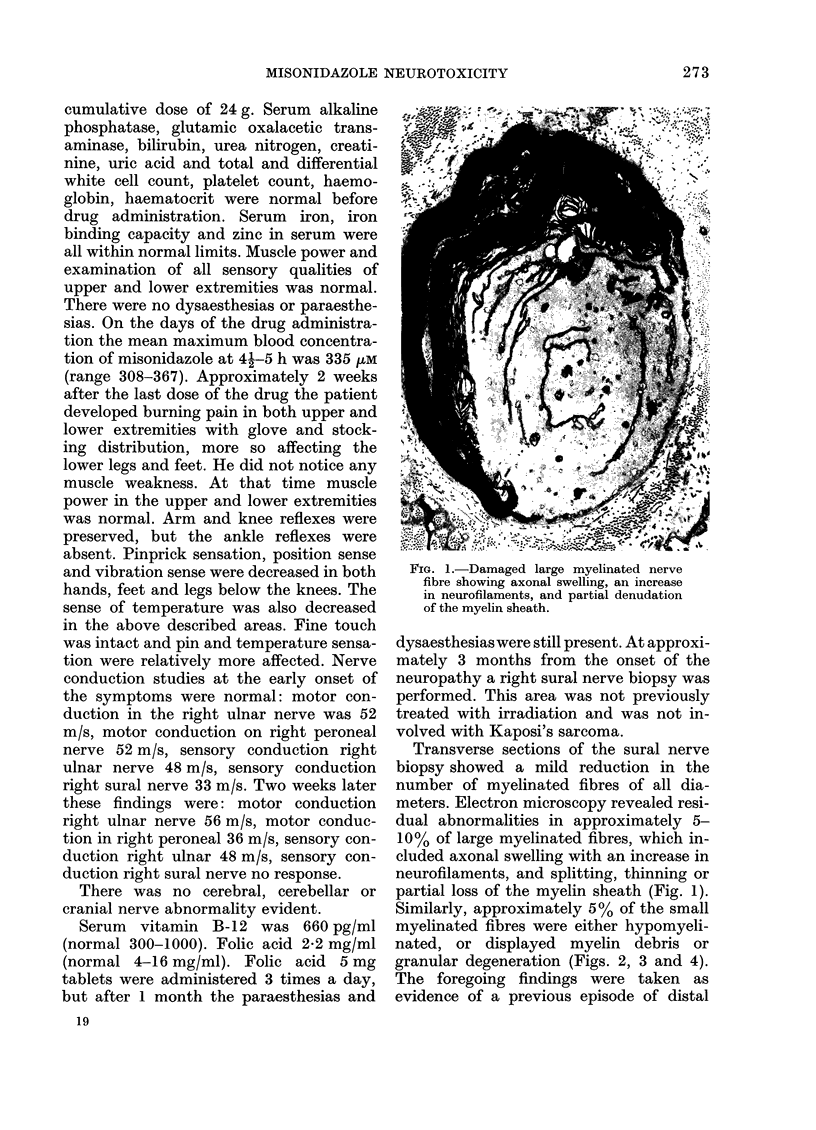
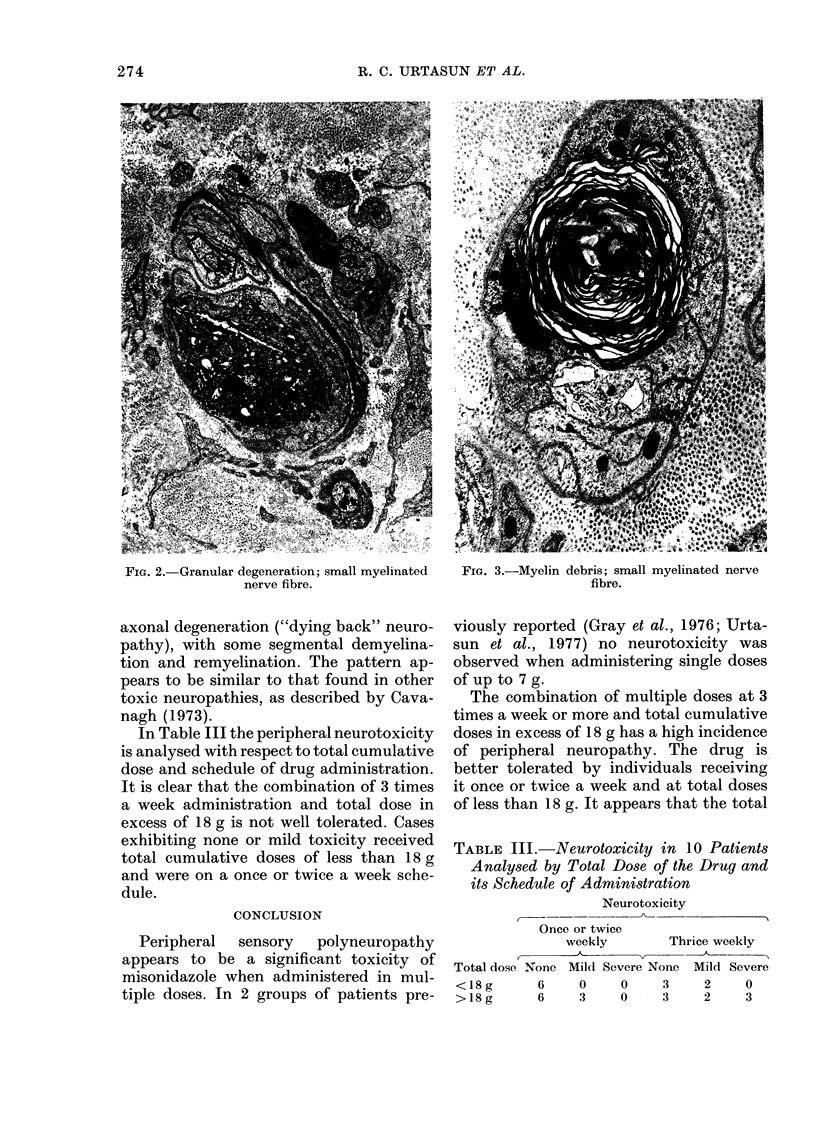
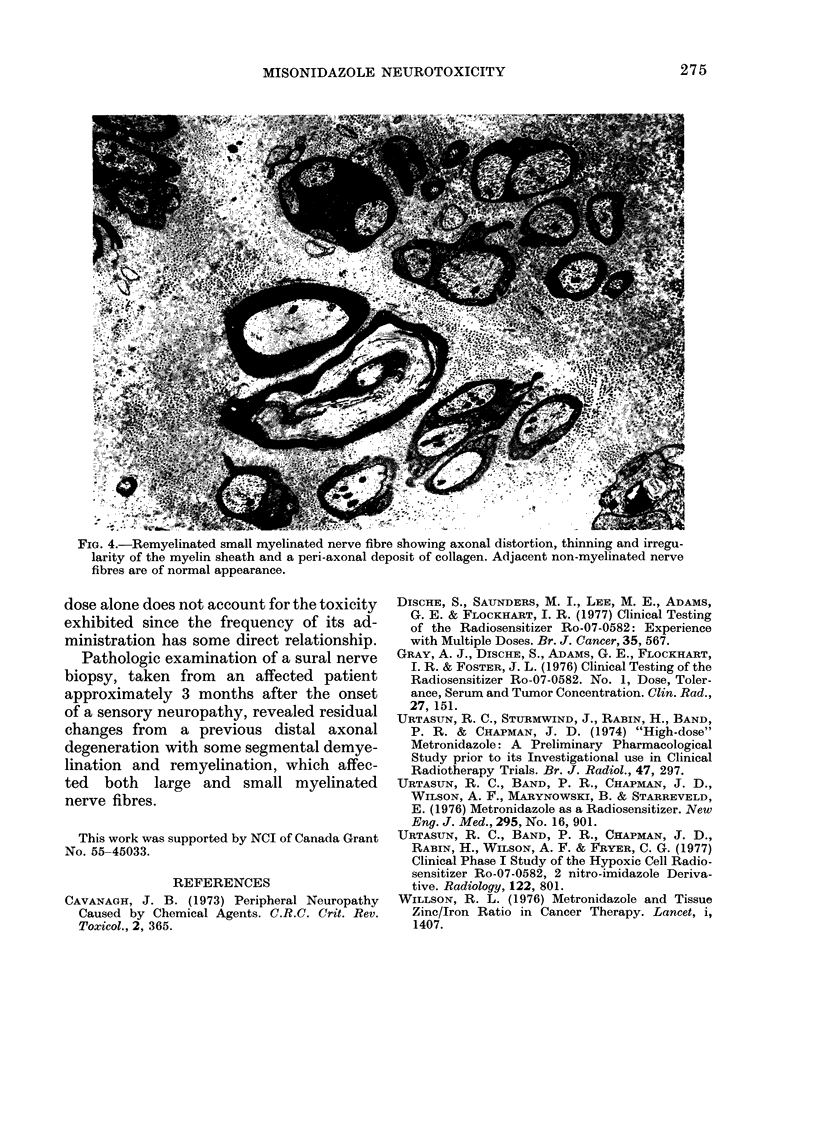
The human tolerance to multiple dosages of misonidazole (Ro-07-0582) was studied in 28 patients with different types of malignant neoplasias. The mean total dose for this group of patients was 16.2 g. The main toxicity was peripheral neuropathy with an overall incidence of 35%. This neuropathy occurred more frequently and with greater severity when the drug was administered 3 times a week and when patients received total doses of over 18 g. The best tolerated schedule appears to be once or twice a week up to total dosages of 18 g or less (approximately 11 g/m2). Electron microscopy of a sural nerve biopsy from an affected patient revealed residual of previous distal axonal degeneration, with some segmental demyelination and remyelination, which affected both large and small myelinated nerve fibres.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavanagh J. B. Peripheral neuropathy caused by chemical agents. CRC Crit Rev Toxicol. 1973 Nov;2(3):365–417. doi: 10.3109/10408447309082021. [DOI] [PubMed] [Google Scholar]
- Dische S., Saunders M. I., Lee M. E., Adams G. E., Flockhart I. R. Clinical testing of the radiosensitizer Ro 07-0582: experience with multiple doses. Br J Cancer. 1977 May;35(5):567–579. doi: 10.1038/bjc.1977.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A. J., Dische S., Adams G. E., Flockhart I. R., Foster J. L. Clinical testing of the radiosensitiser Ro-07-0582. I. Dose tolerance, serum and tumour concentrations. Clin Radiol. 1976 Apr;27(2):151–157. doi: 10.1016/s0009-9260(76)80137-7. [DOI] [PubMed] [Google Scholar]
- Urtasun R. C., Band P., Chapman J. D., Rabin H. R., Wilson A. F., Fryer C. G. Clinical phase I study of the hypoxic cell radiosensitizer RO-07-0582, a 2-nitroimidazole derivative. Radiology. 1977 Mar;122(3):801–804. doi: 10.1148/122.3.801. [DOI] [PubMed] [Google Scholar]
- Urtasun R. C., Sturmwind J., Rabin H., Band P. R., Chapman J. D. Letter: "High-dose" metronidazole: a preliminary pharmacological study prior to its investigational use in clinical radiotherapy trials. Br J Radiol. 1974 May;47(557):297–299. doi: 10.1259/0007-1285-47-557-297-c. [DOI] [PubMed] [Google Scholar]
- Willson R. L. Letter: Metronidazole and tissue zinc/iron ratio in cancer therapy. Lancet. 1976 Jun 26;1(7974):1407–1407. doi: 10.1016/s0140-6736(76)93053-1. [DOI] [PubMed] [Google Scholar]