Abstract
The mesolimbic dopamine system has recently been implicated in the long-term aversive consequences of withdrawal from major drugs of abuse. In the present study we sought to determine whether mesolimbic dopamine neurons are involved in the neurobiologic mechanisms underlying withdrawal from chronic cannabinoid exposure. Rats were treated chronically with the major psychoactive ingredient of hashish and marijuana, Δ9-tetrahydrocannabinol (Δ9-THC). Administration of the cannabinoid antagonist SR 141716A precipitated an intense behavioral withdrawal syndrome, whereas abrupt Δ9-THC suspension failed to produce overt signs of abstinence. In contrast, both groups showed a reduction in dopamine cells activity as indicated by extracellular single unit recordings from antidromically identified meso-accumbens dopamine neurons. The administration of Δ9-THC to spontaneously withdrawn rats restored neuronal activity. Conversely, SR 141716A produced a further decrease of spontaneous activity in cannabinoid-treated although it was ineffective in control rats. These data indicate that withdrawal from chronic cannabinoid administration is associated with reduced dopaminergic transmission in the limbic system, similar to that observed with other addictive drugs; these changes in neuronal plasticity may play a role in drug craving and relapse into drug addiction.
Popular derivatives of the hemp plant (Cannabis sativa) such as hashish and marijuana are the most abused substances among illicit compounds currently available for recreational use (1). Much of this popularity may stem from the fact that socially they are widely tolerated and often perceived as harmless and only mildly addictive (2). Probably because of their long half-lives in the body (3, 4) it has been traditionally difficult to demonstrate a well-defined withdrawal syndrome that, in turn, is one of the qualifying elements in the recognition and classification of drugs of abuse (5). Another factor that may be viewed as important in the clarification of these issues has been the absence of pharmacological tools (antagonists) appropriate for these investigations. Recently, however, SR 141716A, a compound with antagonistic properties toward the central cannabinoid receptor (CB1), has been described (6) and it has made possible to ascribe specific effects to the stimulation of CB1 receptors. In line with this, it has been shown that the blockade of chronic stimulation of CB1 receptors with SR 141716A, precipitates a behavioral withdrawal syndrome (7, 8) that is accompanied by a marked elevation of corticotropin-releasing factor in the limbic system (9). This effect, previously observed after withdrawal from other abused substances, is thought to subserve the neuronal mechanisms of stress and anxiety (10) that are part of the negative emotional state common to withdrawal from drugs of abuse.
The mesolimbic dopamine (DA) system, aside from its recognized role in the acute rewarding effects of addictive substances, has recently been implicated in the long-term aversive consequences of spontaneous and drug-induced withdrawal from major drugs of abuse such as ethanol, morphine and cocaine (11, 12). Cannabinoids, although widely abused by humans, are traditionally considered somewhat milder and less addictive than other drugs of abuse. Nevertheless, they are known to produce, upon cessation of chronic intake, a somatic withdrawal syndrome, and when acutely administered, to stimulate DA neuronal activity in the limbic system (13–15) two effects recognized to be peculiar to major addictive drugs (14, 16, 17).
More relevant to the issue of drug addiction is, however, the involvement of the dopaminergic system in the chronic effects of addictive drugs. In line with this, it was found that the spontaneous activity of DA neurons of the meso-accumbens pathway is reduced after withdrawal from chronic administration of ethanol (18), and morphine (19), and that the reduction in their activity persists after somatic withdrawal has subsided (12, 20). These findings are consistent with the idea that mesolimbic DA participates in the long-term aversive consequences of drug withdrawal, which may contribute to drug seeking behavior and relapse into drug dependence.
We report here that chronic treatment with cannabinoid, and subsequent withdrawal, results in a reduction in the neuronal activity of the mesolimbic dopaminergic pathway independent of somatic manifestations of abstinence.
METHODS
Subjects and Cannabinoid Treatment.
Male Sprague–Dawley albino rats (200–300 g) were used in all experiments. All subjects were kept on a 12 h/12 h light/dark cycle with food and water available ad libitum. Experimental protocols were approved by the Ethical Committee at the University of Cagliari and performed in strict accordance with the European Community regulations for the use of experimental animals (CEE N°86/609). Δ9-Tetrahydrocannabinol (Δ9-THC), in ethanol solution, was purchased from Research Biochemicals International (Natick, MA); it was evaporated immediately before use under argon gas and the residue was emulsified in 1% Tween 80, then diluted in a saline solution. SR 141716A was obtained through Sanofi Research (Paris) and emulsified in 1% Tween 80, then diluted in a saline solution. Drugs were administered i.p. in a volume of 3 ml per kg of body weight. Animals assigned to the cannabinoid withdrawal condition (n = 20) received twice daily injections of Δ9-THC (15 mg/kg i.p.) for 6.5 days; a subgroup of these animals (n = 10) received SR 141716A (10 mg/kg i.p.). 10 min after administration of SR 141716A, or its vehicle, rats were placed singly in Plexiglass cages (30 × 25 × 45 cm) with standard rat litter on the floor. Cages were located in a sound-proof room for behavioral observation. Point-scoring was performed by an observer blind to treatment placed behind a one-way window. Cannabinoid withdrawal signs in both spontaneous and SR 141716A-induced withdrawal were evaluated with counted signs (total number of events over a 30 min period of time) such as scratching, wet dog shakes, facial rubbing, and licking. Counted signs were subjected to nonparametric statistical analysis (Mann–Whitney U test).
Electrophysiological Recording.
Standard extracellular recordings from antidromically identified ventral tegmental area (VTA)-accumbens dopaminergic neurons were performed as already described (18) with the exception that gallamine triethiodide (20 mg/kg i.v.) was used as a muscle relaxant instead of d-tubocurarine. The choice of locally anesthetized rats is based on the fact that general anesthesia (chloral hydrate, ketamine) has been shown to alter the effect of various cannabinoids on the electrophysiological response of mesolimbic dopaminergic neurons (15, 21). Briefly, subjects were temporarily anesthetized with halothane/room air inhalation anesthesia and the femoral vein was cannulated for i.v. administration of pharmacological agents. The trachea was then exposed and incised to allow tracheal intubation with a Teflon catheter for artificial respiration. Gallamine triethiodide (20 mg/kg i.v.) was administered i.v. and once muscular paralysis was obtained the rat was placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA) and the tracheal catheter connected with a mechanical rodent ventilator (7025 Stoelting) set to deliver 90 strokes/min (3 ml/stroke). Incision and pressure points were infiltrated with a 2% solution of xylocaine. Body temperature was maintained constant at 38 ± 1°C by means of an electrically controlled heating pad. Thereafter, rats were prepared as follows: the scalp was retracted and two burr holes were drilled: one for the placement of a recording electrode above the VTA (AP 1.8–2.0 from lambda; L 0.2–0.6 from midline) (22), and the other to introduce a Formvar coated stainless-steel bipolar electrode for antidromic identification in the nucleus accumbens (AP 8.8 from lambda; L 1.6 from midline; V 7.1 from dura matter) (22, 23). Stimuli consisting of monophasic rectangular pulses (0.1–2.0 mA; 0.1–0.5 msec; 0.8 Hz) were generated. Stimulating current was monitored on the oscilloscope. Dopaminergic neurons were identified according to well established electrophysiological characteristics [i.e., (ì) Action potentials with biphasic or triphasic waveforms greater than 2.5 msec in duration. (ìì) A typically slow spontaneous firing rate. (ììì) Occurrence of single and burst spontaneous firing pattern. (ìv) Long antidromic latency] and by antidromic activation from the nucleus accumbens including collision of an antidromically elicited spike with spontaneously occurring action potentials (23). The extracellular neuronal signal from single neurons was amplified (Neurolog System, Digitimer, Welwyn Garden City, U.K.) and displayed on a digital oscilloscope (Philips pm 3305) before storage on magnetic tape for off-line analysis of the data. Firing rate and pattern analysis were performed as already described (24). The basal firing rate was recorded for 5 min. The number of spontaneously active DA neurons was determined by passing the electrode eight times through four different areas within the VTA, according to the method proposed by Bunney and Grace (25). Coordinates of tracks were 2.0 and 2.2 mm anterior to lambda and 0.3 and 0.5 mm lateral to midline (22). The electrode was lowered twice in each spot, whereas the total number of active cells were counted and divided by the number of passes to obtain cells/track index. The statistical significance of the data was evaluated by using one-way ANOVA + Newman-Keuls’ test.
RESULTS
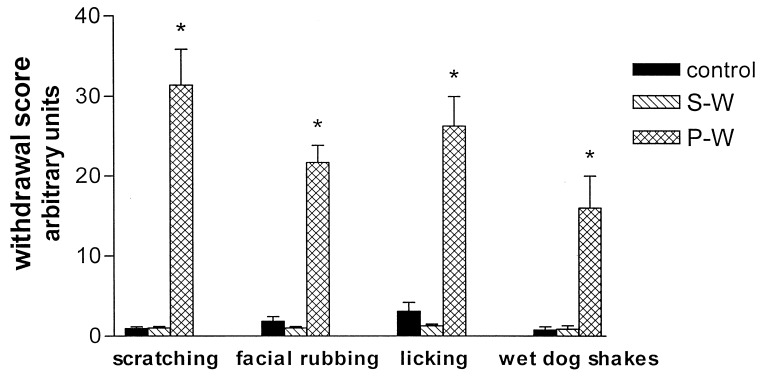
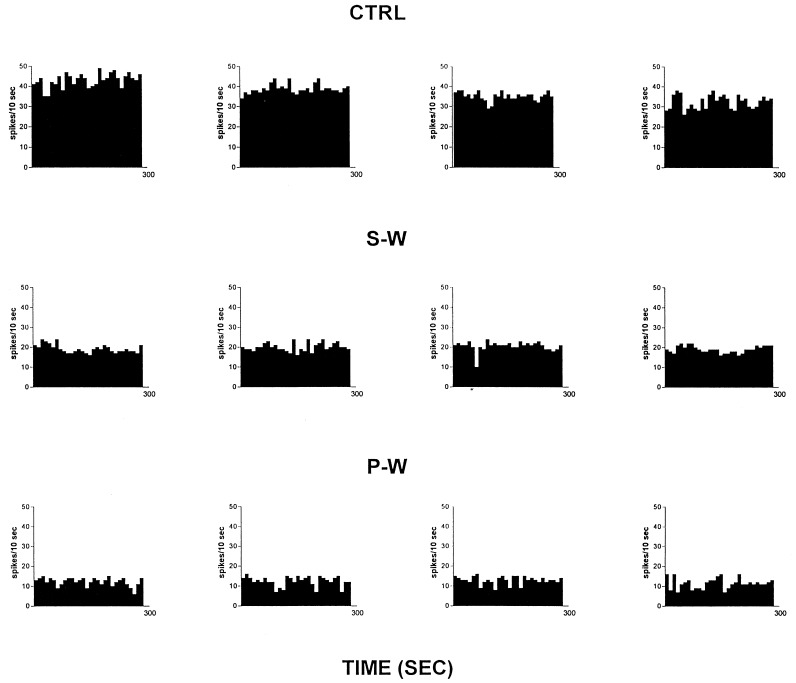
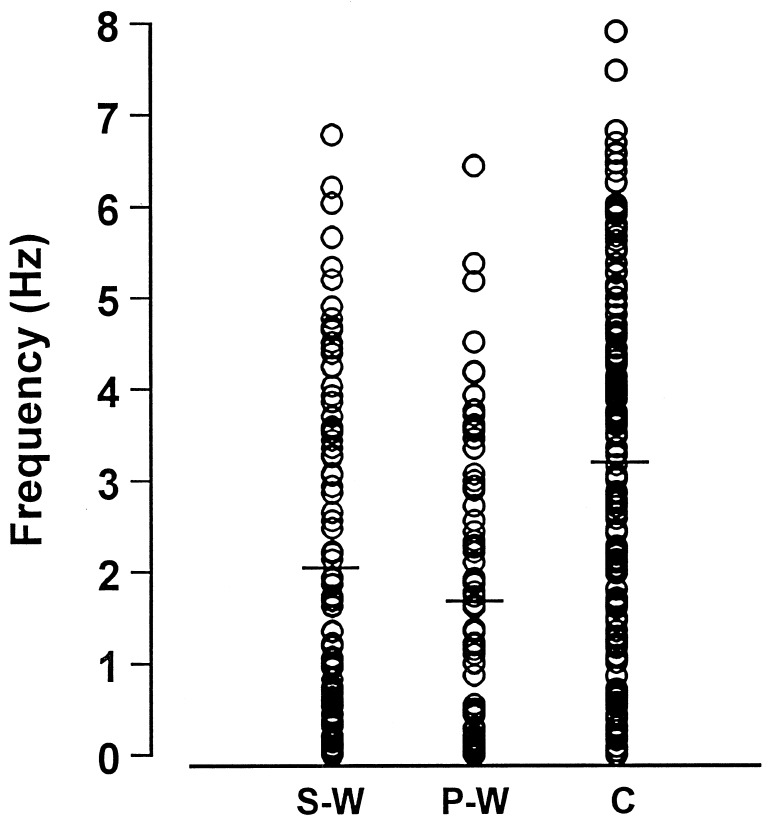
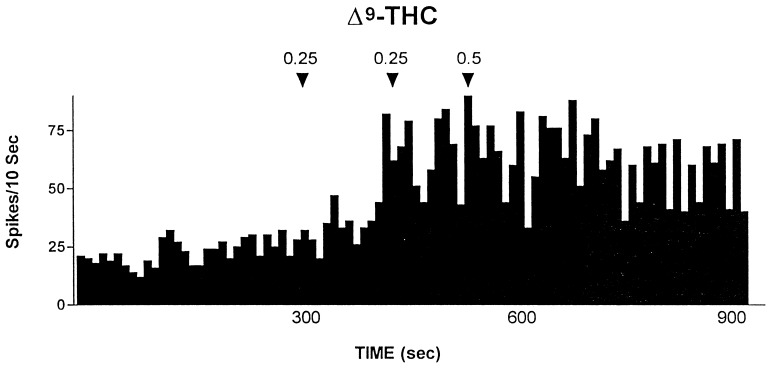
Rats treated twice daily for 6.5 days at the dose of 15 mg/kg i.p., 24 h after the last cannabinoid administration were evaluated behaviorally (Fig. 1). Pharmacologically induced withdrawal (P-W), precipitated by administration of the CB1 antagonist SR 141716A (10 mg/kg i.p.; n = 10), produced an intense behavioral manifestation similar to previous observations (7, 8), whereas spontaneous (S-W) (n = 10) failed to produce overt signs of withdrawal. In fact, when compared with control rats (Fig. 1) S-W rats were found not statistically different (P > 0.05 Mann–Whitney U test). After the 30 min behavioral observation, rats were prepared for standard extracellular recording experiments. VTA DA neurons were antidromically identified from the nucleus accumbens (22, 23) and were found to have altered basal electrophysiological characteristics in respect to corresponding vehicle-treated controls (Table 1). In particular, firing rates (as depicted in Figs. 2, 3, 4), burst firing and spikes/burst of individual neurons were found to be below control levels in both groups (Table 1). Intravenous administration of Δ9-THC (0.25–1 mg/kg) to a subgroup of S-W rats (n = 4) restored the declining dopaminergic electrical activity (Fig. 4). Percentage increase was 159.04 ± 23.73 (n = 4, P < 0.01, Student’s t test) when compared with pre-drug baseline. However, if compared with the mean firing rate of control rats (table 1), the increase in firing rate was found to be not significantly different (65 ± 23.64%; n = 4, P > 0.05 Student’s t test for unpaired observations). Conversely, the administration of SR 141716A, to another subgroup of S-W rats (n = 3), resulted in a marked and long-lasting reduction of DA neuronal activity (Fig. 5A). Percentage decrease in firing rate produced by the cannabinoid antagonist SR 141716A was 49.1 ± 8.7% when compared with pre-drug baseline (P < 0.01, Student’s t test). In contrast, administration of SR 141716A (n = 5), at the same dosage, to control (vehicle-treated) rats did not alter (0.6 ± 0.14%; P > 0.05 Student’s t test for unpaired observations vs. pre-drug baseline) firing rate of mesolimbic DA neurons (Fig. 5B).
Figure 1.
Mean ± SEM of summed behavioral cannabinoid-withdrawal scores observed for a total of 30-min observation period before electrophysiological experiments. Control (vehicle-treated n = 18). P-W rats (n = 10) treated for 6.5 days with Δ9-THC and challenged with the cannabinoid antagonist SR141716A (10 mg/kg i.p.). S-W rats (n = 10) treated for 6.5 days with Δ9-THC and challenged with vehicle. Control and S-W groups are not statistically different (Mann–Whitney U test, U: 79–84.5, P > 0.2). Conversely, P-W rats show a marked behavioral withdrawal syndrome whose score is statistically significant (Mann–Whitney U test, U: 69.5, P < 0.001) when compared with the S-W group.
Table 1.
Various electrophysiological parameters recorded from Δ9-THC withdrawn, both spontaneous (S-W) and induced (P-W), and vehicle-treated (CTRL) rats
| No. of cells | Cells/track | Firing rate, Hz | Burst rate, burst/sec | Spikes/burst | |
|---|---|---|---|---|---|
| CTRL | 142 | 0.99 ± 0.06 | 3.2 ± 0.07 | 0.33 ± 0.02 | 3.26 ± 0.05 |
| S-W | 96 | 1.2 ± 0.08 | 2.05 ± 0.07** | 0.14 ± 0.02** | 2.62 ± 0.07* |
| P-W | 90 | 1.12 ± 0.05 | 1.68 ± 0.04** | 0.13 ± 0.01** | 2.15 ± 0.09** |
Cells/track, number of cells responding to antidromic identification from nucleus accumbens divided by the number of tracks; firing rate, number of spikes per second, Hz; burst rate, number of bursts averaged for 1 sec; spikes/burst, number of action potentials per each burst. Statistical analysis (ANOVA + Newman–Keuls’ test) revealed that Δ9-THC withdrawn rats showed a reduced spontaneous activity in terms of firing rate [F(2,35) = 16.53, **P < 0.01], burst firing [F(2,35) = 7.06; **P < 0.01] and spikes/burst [F(2,35) = 8.28; *P < 0.05, **P < 0.01].
Figure 2.
Spontaneous activity of antidromically identified VTA-accumbens DA neurons encountered in a rat belonging to the CTRL (Top) S-W (Middle) and P-W (Bottom) groups, respectively. Each histogram represents the neuronal activity of a single neuron. Note the difference in spontaneous activity in the three groups. Recordings were obtained 24 h after last Δ9-THC (S-W) and vehicle administration (CTRL) and 2–3 h after SR 141716A administration (P-W).
Figure 3.
Individual firing rates of antidromically identified VTA-accumbens DA neurons recorded from S-W, P-W and Control groups. Each circle represents the mean firing rate of 5-min recording. Note the difference in distribution and the trend in silencing from Control toward cannabinoid withdrawal conditions. Horizontal bars indicate the mean activity as reported in Table 1.
Figure 4.
Example of the effect of cumulative doses of the cannabinoid agonist Δ9-THC administered i.v. at arrows on an antidromically identified VTA-accumbens DA neuron recorded from a S-W rat. Numbers above arrows indicate dosage expressed in mg/kg. Recording was performed 24 h after last Δ9-THC administration.
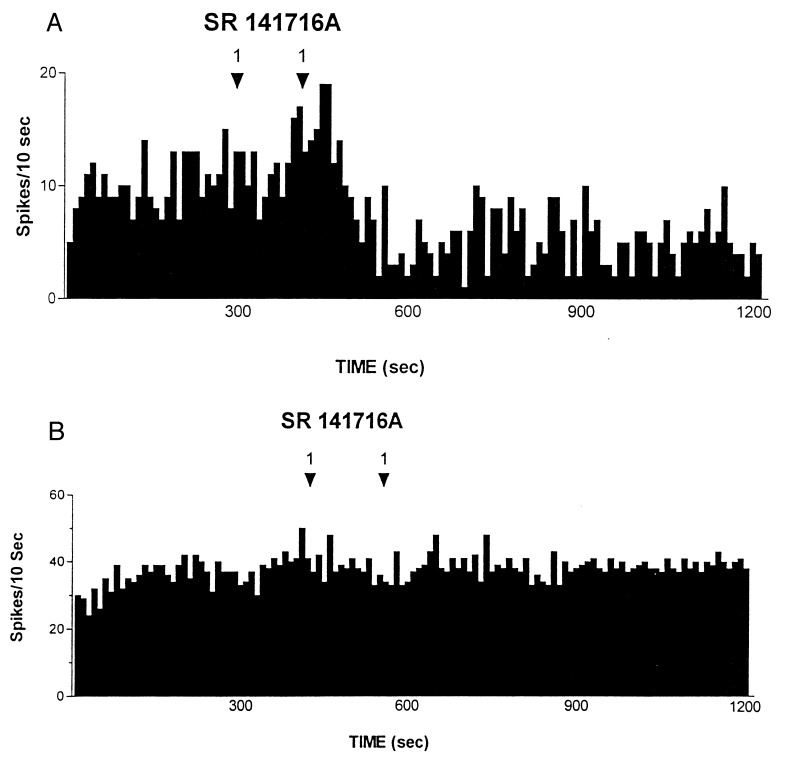
Figure 5.
(A) Effect of the cannabinoid antagonist SR 141716A administered i.v. at arrows on an antidromically identified VTA-accumbens DA neuron recorded from a rat belonging to the S-W group. Recording was performed 24 h after last Δ9-THC administration. (B) The figure shows the failure of i.v. SR 141716A, administered at arrows, in altering the spontaneous neuronal activity of an antidromically identified VTA-accumbens DA neuron recorded from a rat belonging to the Control group. Recording was performed 24 h after last vehicle administration. Numbers above arrows indicate dosage expressed in mg/kg.
DISCUSSION
Collectively, these results indicate that withdrawal from chronic treatment with Δ9-THC induces changes in neuronal plasticity that can be further unmasked by administration of the specific cannabinoid antagonist SR 141716A. Indeed, rats undergoing S-W from Δ9-THC do not show a somatic manifestation of the withdrawal syndrome as observed in their SR 141716-treated (P-W) counterparts. Nevertheless, both groups (SR 141716A-treated and untreated) showed a decline in the dopaminergic functioning of the mesolimbic system of similar magnitude (Table 1). This result indicates that reduction in electrophysiological activity of DA neurons antidromically identified as projecting to the nucleus accumbens, has no relationship with behavioral manifestations of cannabinoid withdrawal. Interestingly, this lack of relationship has been already documented for two major addictive drugs such as ethanol (12, 20) and morphine (12). Further, Δ9-THC administration to rats not exposed to the antagonist resulted in increased firing rates comparable to those observed in controls (15), whereas SR 141716A administration resulted in an abrupt reduction in the already abated electrophysiological activity of DA neurons (Fig. 5A). Because SR 141716A was ineffective when administered to control rats, these results indicate that DA neurons of the limbic system, after chronic Δ9-THC, are driven in their spontaneous activity by the exogenous cannabinoid. In addition, the lack of effect of SR 141716A in control rats (see also ref. 15, 27), suggests that endogenous cannabinoids are unlikely to participate in the modulation of electrophysiological activity of DA neurons of the mesolimbic system. Increased firing rate after i.v. Δ9-THC in S-W rats, together with the abrupt reduction in mesolimbic DA neuronal activity observed after administration of the cannabinoid antagonist SR 141716A in S-W rats but not in controls, suggest that these changes are ascribable to withdrawal from chronic Δ9-THC.
These changes in the dopaminergic functioning of the limbic system, affecting specifically firing rate, burst firing and spikes/burst of these units, with no alteration in the cells/track index, suggest that VTA DA neurons projecting to the nucleus accumbens are reduced in their spontaneous activity but not affected by depolarization blockade (26). Thus they appear to be more refractory to intrinsic and extrinsic depolarizing stimuli. However, irrespective of the mechanism underlying the reduction in dopaminergic neuronal function, the present results indicate that withdrawal from chronic cannabinoid exposure induces subtle changes in the mesolimbic dopaminergic system that can be further evidenced by administration of SR 141716A, per se ineffective (15, 27). These changes, indexed by neurophysiological and pharmacological evidences, are: (i) reminiscent of those recently reported for other, more harmful, substances such as ethanol and opiates (12, 18, 28). Indeed, if compared, the reduction in DA neuronal activity is qualitatively, but not quantitatively, similar in the three withdrawal syndromes, being morphine>cannabinoids>ethanol, respectively. (ii) Consistent with the idea that lessening dopaminergic functioning in the limbic system may substantially contribute to the neurobiological basis of the subjective aversive consequences of drug withdrawal (dysphoria) rather than somatic aspects of it (29).
These data are part of a large and still growing body of evidence that indicates that chronic administration of addictive drugs results in major changes in the physiology (11, 12), biochemistry (30, 31) and even anatomy (32) of the mesolimbic DA system. These changes, in turn, may render the mesolimbic DA pathway more vulnerable and thus facilitate relapse into a drug dependent state. From another viewpoint, these data indicate that withdrawal from chronic cannabinoid exposure results in neurobiological alterations not dissimilar from those observed after protracted administration with substances, such as ethanol and opiates, which are widely considered to be more harmful.
Acknowledgments
We thank Mr. S. Aramo for his skillful technical assistance and Sanofi Research for the gift of SR 141716A.
ABBREVIATIONS
- Δ9-THC
Δ9-tetrahydrocannabinol
- DA
dopamine
- VTA
ventral-tegmental area
- P-W
pharmacologically induced withdrawal
- S-W
spontaneous withdrawal
References
- 1.Martin B R. In: Marijuana in Psychopharmacology: The Fourth Generation of Progress. Bloom F E, Kupfer D J, editors. New York: Raven; 1995. pp. 1757–1765. [Google Scholar]
- 2.Grinspoon L, Bakalar J B. In: Substance Abuse, A Comprehensive Textbook. 2nd Ed. Lowinson J H, Ruiz P, Millman R B, editors. Baltimore: Williams & Wilkins; 1992. pp. 236–246. [Google Scholar]
- 3.Truitt E B., Jr Pharmacol Rev. 1971;23:273–278. [PubMed] [Google Scholar]
- 4.Lemberger L, Axelrod J, Kopin I J. Pharmacol Rev. 1971;23:371–380. [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Ed. Washington, D.C.: Am. Psychiatr. Assoc.; 1994. [Google Scholar]
- 6.Rinaldi-Carmona M, Barth F, Hèaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Nèliat G, Caput D, et al. FEBS Letts. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 7.Tsou K, Patrick S L, Walker J M. Eur J Pharmacol. 1995;280:R13–R15. doi: 10.1016/0014-2999(95)00360-w. [DOI] [PubMed] [Google Scholar]
- 8.Aceto M D, Scates S M, Lowe J A, Martin B R. Eur J Pharmacol. 1995;282:R1–R2. doi: 10.1016/0014-2999(95)00447-s. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez de Fonseca F, Carrera M R A, Navarro M, Koob G F, Weiss F. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- 10.Koob G F. Neuron. 1996;16(5):893. doi: 10.1016/s0896-6273(00)80109-9. [DOI] [PubMed] [Google Scholar]
- 11.White F J. Annu Rev Neurosci. 1996;19:405–436. doi: 10.1146/annurev.ne.19.030196.002201. [DOI] [PubMed] [Google Scholar]
- 12.Diana M. Adv Behav Biol. 1996;47:123–130. [Google Scholar]
- 13.French E D, Dillon K, Wu X. NeuroReport. 1997;8:649–652. doi: 10.1097/00001756-199702100-00014. [DOI] [PubMed] [Google Scholar]
- 14.French E D. Neurosci Lett. 1997;226:159–162. doi: 10.1016/s0304-3940(97)00278-4. [DOI] [PubMed] [Google Scholar]
- 15.Gessa G L, Melis M, Muntoni A L, Diana M. Eur J Pharmacol. 1998;341:39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- 16.Matthews R T, German D C. Neuroscience. 1984;11:617–625. doi: 10.1016/0306-4522(84)90048-4. [DOI] [PubMed] [Google Scholar]
- 17.Gessa G L, Muntoni F, Collu M, Vargiu L, Mereu G P. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- 18.Diana M, Pistis M, Carboni S, Gessa G L, Rossetti Z L. Proc Natl Acad Sci USA. 1993;90:7966–7969. doi: 10.1073/pnas.90.17.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diana M, Pistis M, Muntoni A L, Gessa G L. J Pharmacol Exp Ther. 1995;272:781–785. [PubMed] [Google Scholar]
- 20.Diana M, Pistis M, Muntoni A L, Gessa G L. Neuroscience. 1996;71:411–415. doi: 10.1016/0306-4522(95)00482-3. [DOI] [PubMed] [Google Scholar]
- 21.Gifford A N, Gardner E L, Ashby C R. Neuropsychobiology. 1997;36:96–99. doi: 10.1159/000119369. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. London: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 23.Lipsky J. J Neurosci Methods. 1981;4:1–32. doi: 10.1016/0165-0270(81)90015-7. [DOI] [PubMed] [Google Scholar]
- 24.Diana M, Garcia-Munoz M, Richards J B, Freed C R. Exp Brain Res. 1989;74:625–630. doi: 10.1007/BF00247365. [DOI] [PubMed] [Google Scholar]
- 25.Bunney B S, Grace A A. Life Sci. 1978;23:1715–1728. doi: 10.1016/0024-3205(78)90471-x. [DOI] [PubMed] [Google Scholar]
- 26.Diana M, Pistis M, Muntoni A L, Gessa G L. Brain Res. 1995;682:29–34. doi: 10.1016/0006-8993(95)00292-x. [DOI] [PubMed] [Google Scholar]
- 27.Gueudet C, Santucci V, Rinaldi-Carmona M, Soubriè P, Le Fur G. NeuroReport. 1995;6:1293–1297. doi: 10.1097/00001756-199507100-00015. [DOI] [PubMed] [Google Scholar]
- 28.Diana M, Rossetti Z L, Gessa G L. In: Drug Addiction and Related Clinical Problems. Tagliamonte A, Maremmani I, editors. New York: Springer; 1995. pp. 19–26. [Google Scholar]
- 29.Harris H W, Aston-Jones G. Nature (London) 1994;371:155–157. doi: 10.1038/371155a0. [DOI] [PubMed] [Google Scholar]
- 30.Self D W, Nestler E J. Annu Rev Neurosci. 1995;18:463–495. doi: 10.1146/annurev.ne.18.030195.002335. [DOI] [PubMed] [Google Scholar]
- 31.Nestler E J, Hope B T, Widdnell K L. Neuron. 1993;11:995–1006. doi: 10.1016/0896-6273(93)90213-b. [DOI] [PubMed] [Google Scholar]
- 32.Sklair-Tavron Shi, W, Lane S B, Harris H W, Bunney B S, Nestler E J. Proc Natl Acad Sci USA. 1996;93:11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]