Abstract
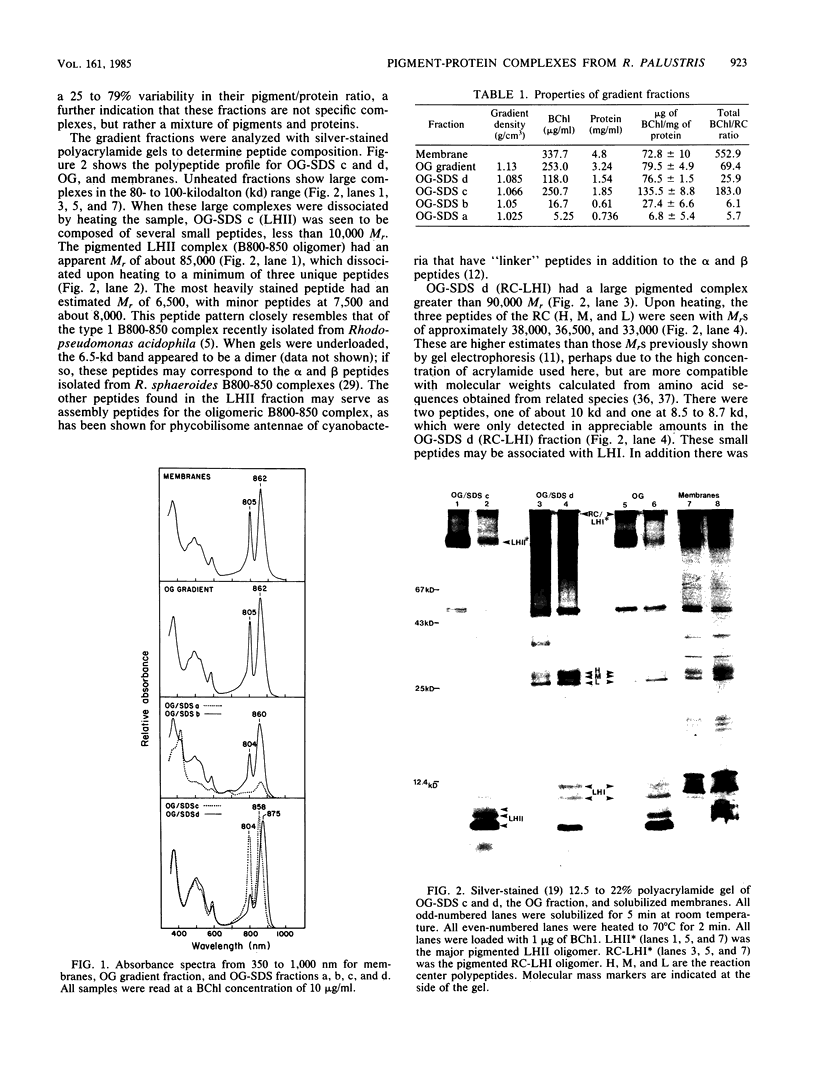
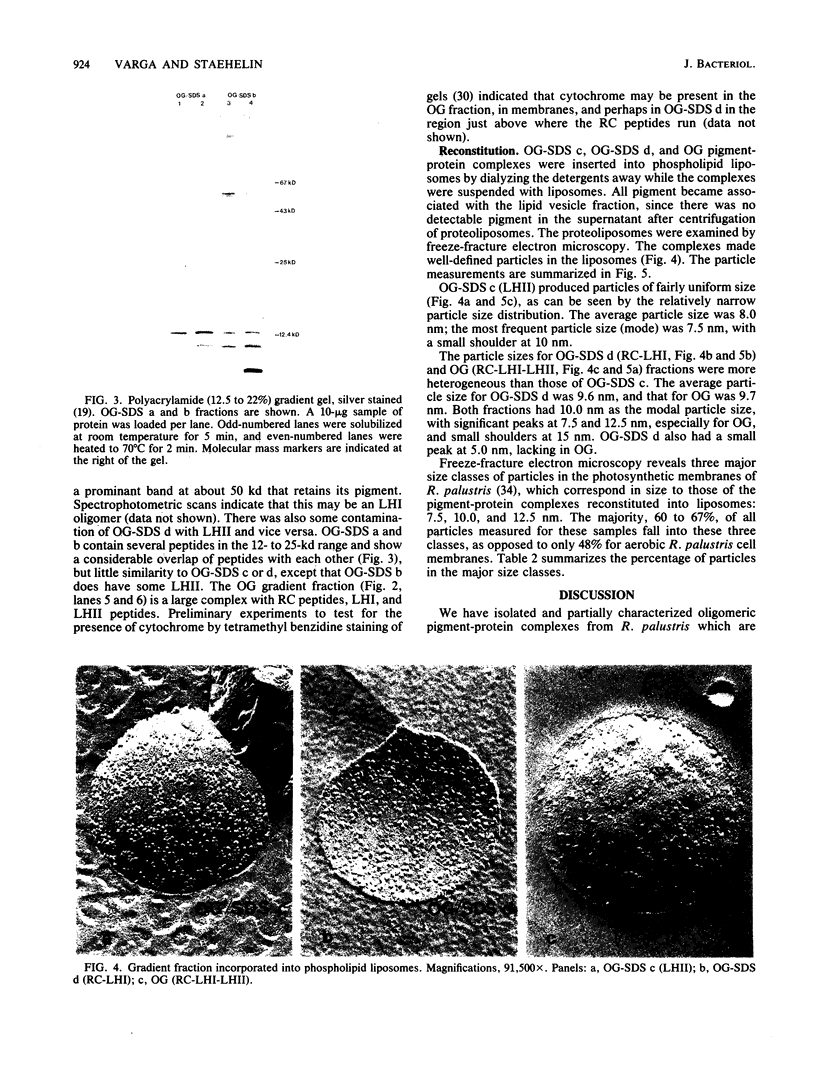
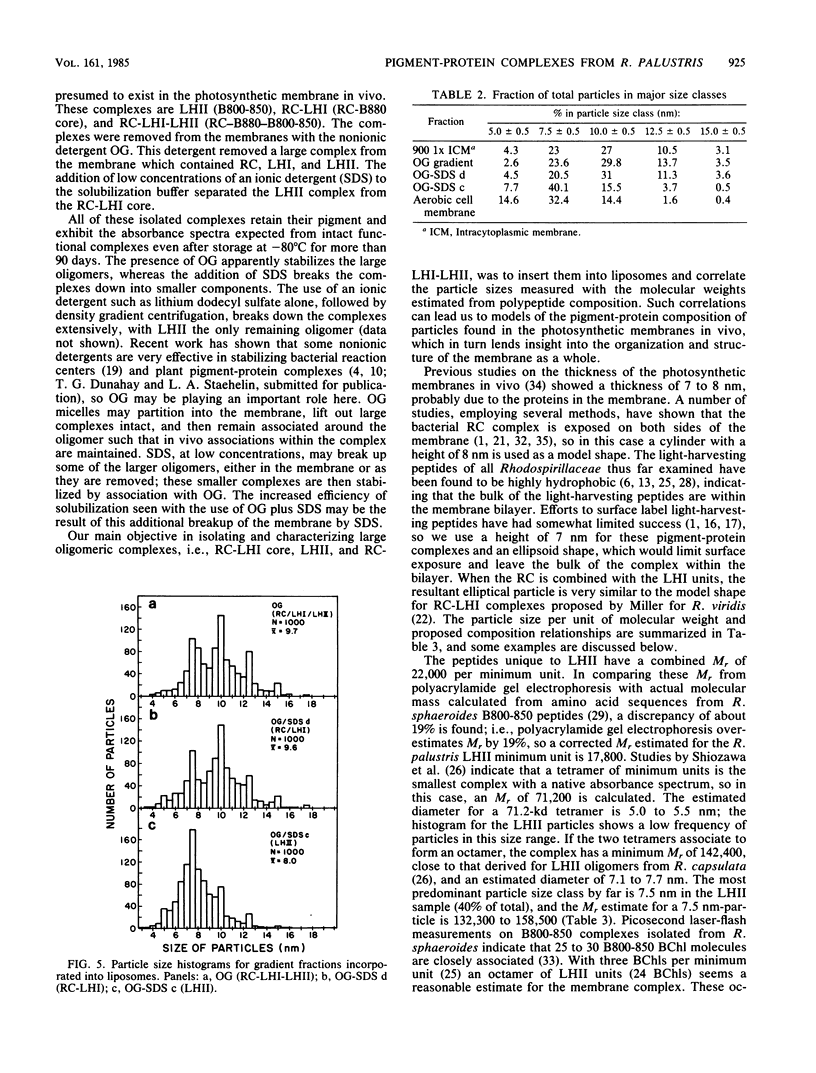
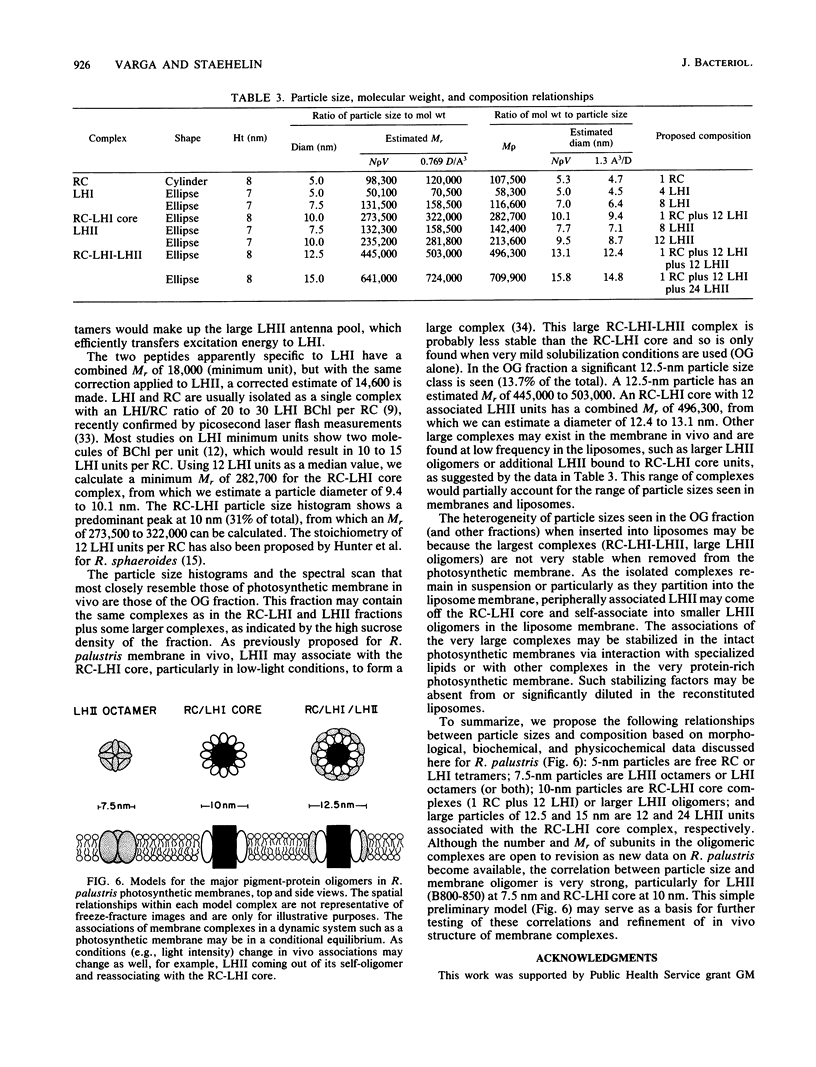
We have employed detergent solubilization and sucrose density gradient centrifugation to obtain pigment-protein complexes from Rhodopseudomonas palustris. Two types of detergent buffers were used, containing either octyl-beta-glucopyranoside (OG) plus sodium dodecyl sulfate (SDS) or OG alone. The fractions thus obtained were analyzed spectrophotometrically and by polyacrylamide gel electrophoresis to determine their pigment and protein composition. OG-SDS solubilization yields four fractions. The least dense of these fractions (OG-SDS a and b) are nonspecific mixtures of peptides and pigments. The next fraction, OG-SDS c, is an accessory light-harvesting complex, LHII, called B800-850. The largest particle, OG-SDS d, is a combination of reaction center (RC) and primary light-harvesting complex (LHI), B880. Solubilization using OG alone yields one fraction, a single large complex consisting of RC, LHI, and LHII. We have inserted the two large OG-SDS complexes and the OG complex into phospholipid liposomes to determine the size of such complexes in freeze-fractured membranes. On the basis of morphological, biochemical, and available biophysical data, we propose the following models for pigment-protein complexes in R. palustris membranes: 5-nm particles as free RC or LHI tetramers, 7.5-nm particles as LHI or LHII octamers (or both); 10-nm particles as RC-LHI core complexes (1 RC plus 12 LHI) or large LHII oligomers (or both), and large particles of 12.5 and 15 nm and LHII associated with the RC-LHI core complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann R. C., Gillies K., Takemoto J. Y. Membrane topography of the photosynthetic reaction center polypeptides of Rhodopseudomonas sphaeroides. Biochemistry. 1981 Aug 4;20(16):4590–4596. doi: 10.1021/bi00519a012. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Camm E. L., Green B. R. Fractionation of Thylakoid Membranes with the Nonionic Detergent Octyl-beta-d-glucopyranoside: RESOLUTION OF CHLOROPHYLL-PROTEIN COMPLEX II INTO TWO CHLOROPHYLL-PROTEIN COMPLEXES. Plant Physiol. 1980 Sep;66(3):428–432. doi: 10.1104/pp.66.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. K., Kaplan S. Characterization of the three major intracytoplasmic membrane polypeptides isolated from Rhodopseudomonas sphaeroides. J Biol Chem. 1981 Jun 10;256(11):5909–5915. [PubMed] [Google Scholar]
- Delepelaire P., Chua N. H. Lithium dodecyl sulfate/polyacrylamide gel electrophoresis of thylakoid membranes at 4 degrees C: Characterizations of two additional chlorophyll a-protein complexes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):111–115. doi: 10.1073/pnas.76.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley P. R., Anderson J. M. The light-harvesting chlorophyll a/b-protein complex from barley thylakoid membranes. Polypeptide composition and characterization of an oligomer. Biochim Biophys Acta. 1979 Jan 11;545(1):174–187. [PubMed] [Google Scholar]
- Firsow N. N., Drews G. Differentiation of the intracytoplasmic membrane of Rhodopseudomonas palustris induced by variations of oxygen partial pressure or light intensity. Arch Microbiol. 1977 Dec 15;115(3):299–306. doi: 10.1007/BF00446456. [DOI] [PubMed] [Google Scholar]
- Glazer A. N. Comparative biochemistry of photosynthetic light-harvesting systems. Annu Rev Biochem. 1983;52:125–157. doi: 10.1146/annurev.bi.52.070183.001013. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Nakano M., Morita S. Comparative studies of protein properties and bacteriochlorophyll contents of bacteriochlorophyll-protein complexes from spectrally different types of Rhodopseudomonas palustris. J Biochem. 1982 Dec;92(6):1805–1811. doi: 10.1093/oxfordjournals.jbchem.a134110. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Hunter C. N., Pennoyer J. D., Niederman R. A. Assembly and structural organization of pigment-protein complexes in membranes of Rhodopseudomonas sphaeroides. Prog Clin Biol Res. 1982;102(Pt B):257–265. [PubMed] [Google Scholar]
- Jacob J. S., Miller K. R. Structure of a bacterial photosynthetic membrane. Isolation, polypeptide composition, and selective proteolysis. Arch Biochem Biophys. 1983 May;223(1):282–290. doi: 10.1016/0003-9861(83)90593-3. [DOI] [PubMed] [Google Scholar]
- Jay F., Lambillotte M., Mühlethaler K. Localisation of Rhodopseudomonas viridis reaction centre and light harvesting proteins using ferritin-antibody labelling. Eur J Cell Biol. 1983 Mar;30(1):1–8. [PubMed] [Google Scholar]
- Kendall-Tobias M. W., Seibert M. A rapid procedure for the isolation and purification of photosynthetic reaction centers from Rhodopseudomonas sphaeroides R-26. Arch Biochem Biophys. 1982 Jun;216(1):255–258. doi: 10.1016/0003-9861(82)90210-7. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Niederman R. A., Mallon D. E., Langan J. J. Membranes of Rhodopseudomonas sphaeroides. IV. Assembly of chromatophores in low-aeration cell suspensions. Biochim Biophys Acta. 1976 Aug 13;440(2):429–447. doi: 10.1016/0005-2728(76)90076-1. [DOI] [PubMed] [Google Scholar]
- Rivas E., Reiss-Husson F., le Maire M. Physicochemical properties of detergent-solubilized photochemical reaction centers from two strains of Rhodopseudomonas spheroides. Biochemistry. 1980 Jun 24;19(13):2943–2950. doi: 10.1021/bi00554a020. [DOI] [PubMed] [Google Scholar]
- Shiozawa J. A., Welte W., Hodapp N., Drews G. Studies on the size and composition of the isolated light-harvesting B800-850 pigment-protein complex of Rhodopseudomonas capsulata. Arch Biochem Biophys. 1982 Feb;213(2):473–485. doi: 10.1016/0003-9861(82)90573-2. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A. Reversible particle movements associated with unstacking and restacking of chloroplast membranes in vitro. J Cell Biol. 1976 Oct;71(1):136–158. doi: 10.1083/jcb.71.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros M. H., Suter F., Seydewitz H. H., Witt I., Zuber H., Drews G. Isolation and complete amino-acid sequence of the small polypeptide from light-harvesting pigment-protein complex I (B870) of Rhodopseudomonas capsulata. Eur J Biochem. 1984 Jan 2;138(1):209–212. doi: 10.1111/j.1432-1033.1984.tb07902.x. [DOI] [PubMed] [Google Scholar]
- Theiler R., Suter F., Wiemken V., Zuber H. The light-harvesting polypeptides of Rhodopseudomonas sphaeroides R-26.1. I. Isolation, purification and sequence analyses. Hoppe Seylers Z Physiol Chem. 1984 Jul;365(7):703–719. doi: 10.1515/bchm2.1984.365.2.703. [DOI] [PubMed] [Google Scholar]
- Thomas P. E., Ryan D., Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976 Sep;75(1):168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Valkirs G. E., Feher G. Topography of reaction center subunits in the membrane of the photosynthetic bacterium, rhodopseudomonas sphaeroides. J Cell Biol. 1982 Oct;95(1):179–188. doi: 10.1083/jcb.95.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga A. R., Staehelin L. A. Spatial differentiation in photosynthetic and non-photosynthetic membranes of Rhodopseudomonas palustris. J Bacteriol. 1983 Jun;154(3):1414–1430. doi: 10.1128/jb.154.3.1414-1430.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Ogden R. C., Simon M. I., Feher G. Primary structure of the M subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6505–6509. doi: 10.1073/pnas.80.21.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Bylina E. J., Alberti M., Begusch H., Hearst J. E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell. 1984 Jul;37(3):949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]