Abstract
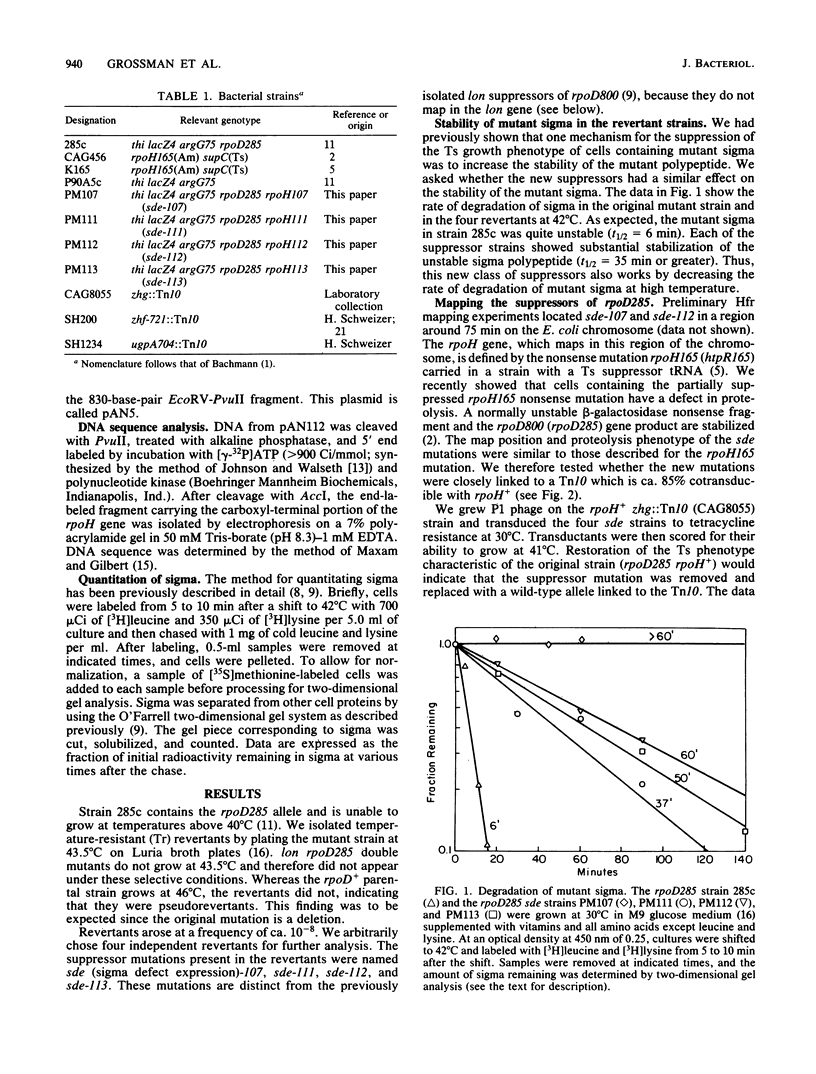
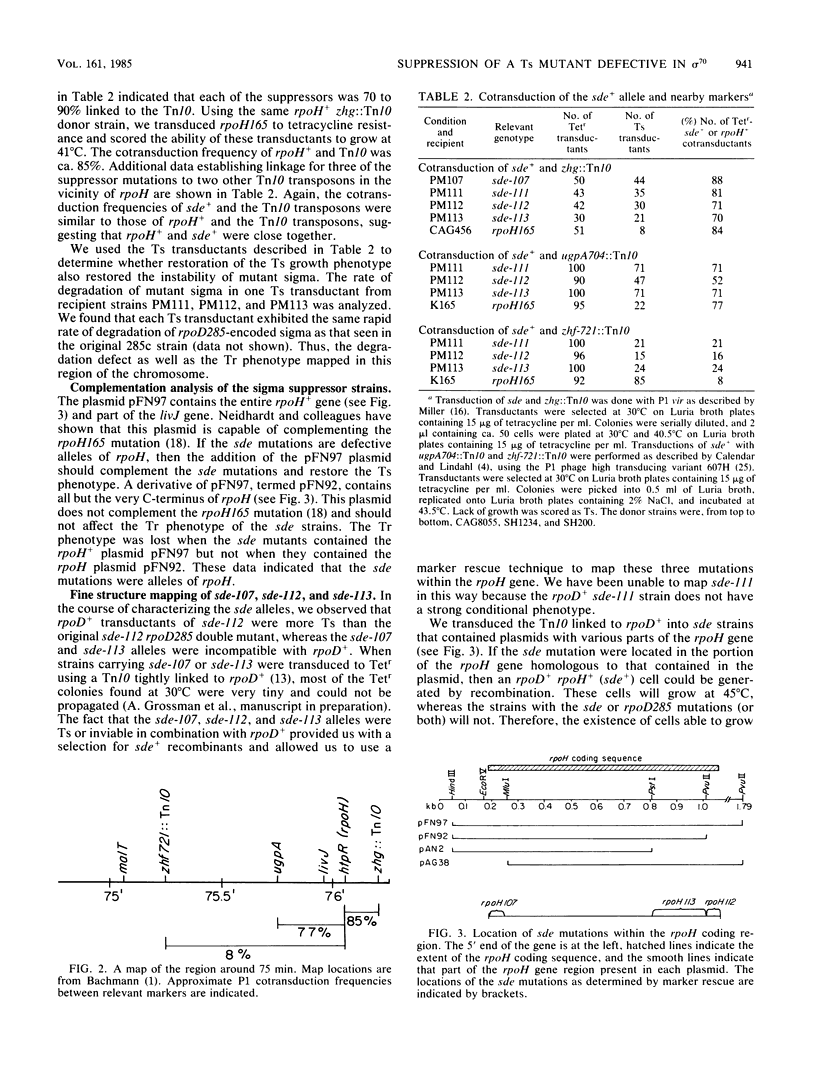
Escherichia coli K-12 strain 285c contains a mutation in rpoD, the gene encoding the sigma subunit of RNA polymerase. The 70-kilodalton sigma polypeptide encoded by this allele is unstable, and this instability leads to temperature-sensitive growth. We describe the isolation and characterization of four temperature-resistant pseudorevertants of 285c that can grow at high temperature. Each of these revertants increased the stability of the sigma 70 mutant protein. The map position of the suppressor mutations was close to that of the rpoH (htpR) gene. A multicopy plasmid containing the intact rpoH gene restored the temperature-sensitive phenotype. Marker rescue experiments established the positions of three of the alleles within the rpoH gene. One mutation has been sequenced and causes a leucine-to-tryptophan change 7 amino acids from the carboxyl terminus of the rpoH gene product.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. A., Grossman A. D., Gross C. A. A gene regulating the heat shock response in Escherichia coli also affects proteolysis. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6779–6783. doi: 10.1073/pnas.81.21.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Calendar R., Lindahl G. Attachment of prophage P2: gene order at different host chromosomal sites. Virology. 1969 Dec;39(4):867–881. doi: 10.1016/0042-6822(69)90023-3. [DOI] [PubMed] [Google Scholar]
- Cooper S., Ruettinger T. A temperature sensitive nonsense mutation affecting the synthesis of a major protein of Escherichia coli K12. Mol Gen Genet. 1975 Aug 5;139(2):167–176. doi: 10.1007/BF00264696. [DOI] [PubMed] [Google Scholar]
- Gillen J. R., Willis D. K., Clark A. J. Genetic analysis of the RecE pathway of genetic recombination in Escherichia coli K-12. J Bacteriol. 1981 Jan;145(1):521–532. doi: 10.1128/jb.145.1.521-532.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Zipser D. Deg phenotype of Escherichia coli lon mutants. J Bacteriol. 1978 Feb;133(2):844–851. doi: 10.1128/jb.133.2.844-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C. A., Grossman A. D., Liebke H., Walter W., Burgess R. R. Effects of the mutant sigma allele rpoD800 on the synthesis of specific macromolecular components of the Escherichia coli K12 cell. J Mol Biol. 1984 Jan 25;172(3):283–300. doi: 10.1016/s0022-2836(84)80027-3. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Burgess R. R., Walter W., Gross C. A. Mutations in the Ion gene of E. coli K12 phenotypically suppress a mutation in the sigma subunit of RNA polymerase. Cell. 1983 Jan;32(1):151–159. doi: 10.1016/0092-8674(83)90505-6. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Erickson J. W., Gross C. A. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell. 1984 Sep;38(2):383–390. doi: 10.1016/0092-8674(84)90493-8. [DOI] [PubMed] [Google Scholar]
- Harris J. D., Heilig J. S., Martinez I. I., Calendar R., Isaksson L. A. Temperature-sensitive Escherichia coli mutant producing a temperature-sensitive sigma subunit of DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6177–6181. doi: 10.1073/pnas.75.12.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. C., Gross C. A. Marker rescue with plasmids bearing deletions in rpoD identifies a dispensable part of E. coli sigma factor. Mol Gen Genet. 1983;191(3):492–498. doi: 10.1007/BF00425768. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Landick R., Vaughn V., Lau E. T., VanBogelen R. A., Erickson J. W., Neidhardt F. C. Nucleotide sequence of the heat shock regulatory gene of E. coli suggests its protein product may be a transcription factor. Cell. 1984 Aug;38(1):175–182. doi: 10.1016/0092-8674(84)90538-5. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Lau E. T. Molecular cloning and expression of a gene that controls the high-temperature regulon of Escherichia coli. J Bacteriol. 1983 Feb;153(2):597–603. doi: 10.1128/jb.153.2.597-603.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A. Positive regulatory gene for temperature-controlled proteins in Escherichia coli. Biochem Biophys Res Commun. 1981 May 29;100(2):894–900. doi: 10.1016/s0006-291x(81)80257-4. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- Osawa T., Yura T. Amber mutations in the structural gene for RNA polymerase sigma factor of Escherichia coli. Mol Gen Genet. 1980;180(2):293–300. doi: 10.1007/BF00425841. [DOI] [PubMed] [Google Scholar]
- Schweizer H., Grussenmeyer T., Boos W. Mapping of two ugp genes coding for the pho regulon-dependent sn-glycerol-3-phosphate transport system of Escherichia coli. J Bacteriol. 1982 Jun;150(3):1164–1171. doi: 10.1128/jb.150.3.1164-1171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe T., Ito K., Yura T. Isolation and physical mapping of temperature-sensitive mutants defective in heat-shock induction of proteins in Escherichia coli. Mol Gen Genet. 1984;195(1-2):10–16. doi: 10.1007/BF00332716. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall J. D., Harriman P. D. Phage P1 mutants with altered transducing abilities for Escherichia coli. Virology. 1974 Jun;59(2):532–544. doi: 10.1016/0042-6822(74)90463-2. [DOI] [PubMed] [Google Scholar]
- Yamamori T., Yura T. Genetic control of heat-shock protein synthesis and its bearing on growth and thermal resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 Feb;79(3):860–864. doi: 10.1073/pnas.79.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]


