Abstract
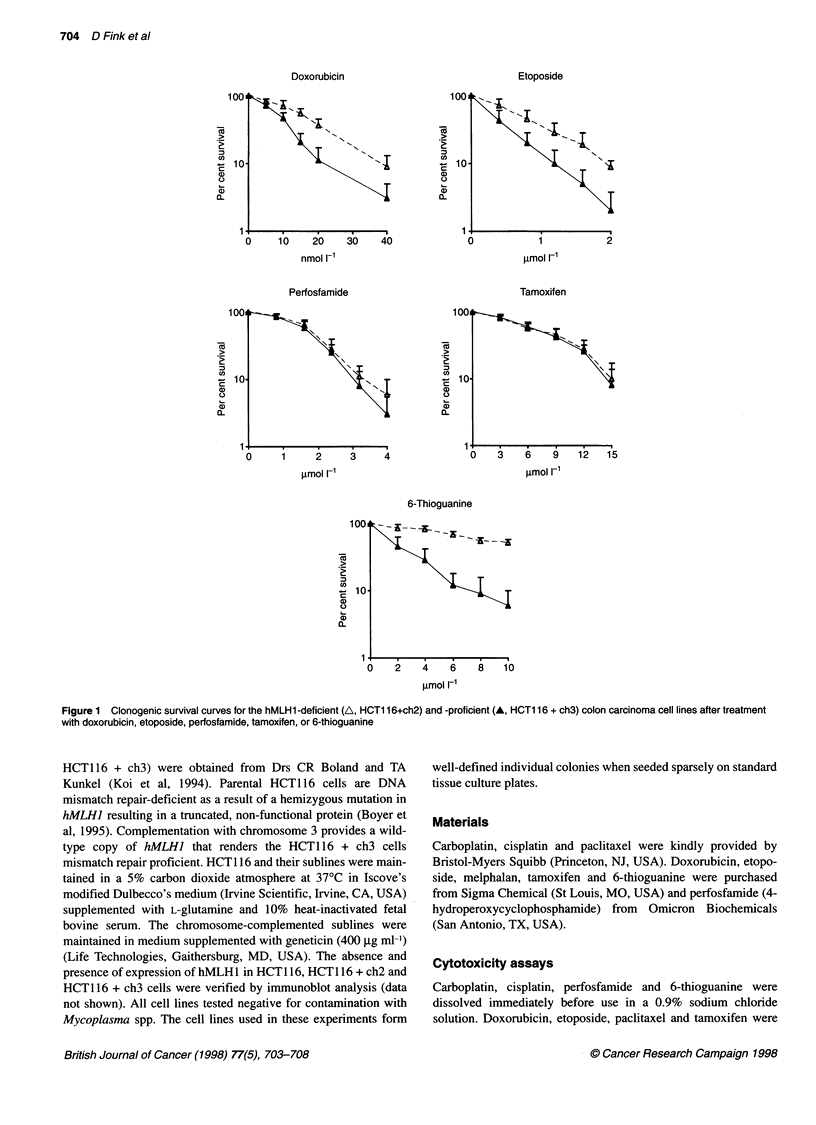
Loss of DNA mismatch repair is a common finding in hereditary non-polyposis colon cancer as well as in many types of sporadic human tumours. We compared the effect of loss of DNA mismatch repair on drug sensitivity as measured by a clonogenic assay with its effect on the ability of the same drug to enrich for mismatch repair-deficient cells in a proliferating tumour cell population. Mixed populations containing 50% DNA mismatch repair-deficient cells constitutively expressing green fluorescent protein and 50% mismatch repair-proficient cells were exposed to different chemotherapeutic agents. 6-Thioguanine, to which DNA mismatch repair-deficient cells are known to be resistant, was included as a control. The results in the cytotoxicity assays and in the enrichment experiments were concordant. Treatment with either carboplatin, cisplatin, doxorubicin, etoposide or 6-thioguanine resulted in enrichment for mismatch repair-deficient cells, and clonogenic assays demonstrated resistance to these agents, which varied from 1.3- to 4.8-fold. Treatment with melphalan, paclitaxel, perfosfamide or tamoxifen failed to enrich for mismatch repair-deficient cells, and no change in sensitivity to these agents was detected in the clonogenic assays. These results identify the topoisomerase II inhibitors etoposide and doxorubicin as additional agents for which loss of DNA mismatch repair causes drug resistance. The concordance of the results from the two assay systems validates the enrichment assay as a rapid and reliable method for screening for the effect of loss of DNA mismatch repair on sensitivity to additional drugs.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya S., Wilson T., Gradia S., Kane M. F., Guerrette S., Marsischky G. T., Kolodner R., Fishel R. hMSH2 forms specific mispair-binding complexes with hMSH3 and hMSH6. Proc Natl Acad Sci U S A. 1996 Nov 26;93(24):13629–13634. doi: 10.1073/pnas.93.24.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi S., Fink D., Gordon R., Kim H. K., Zheng H., Fink J. L., Howell S. B. Resistance to cytotoxic drugs in DNA mismatch repair-deficient cells. Clin Cancer Res. 1997 Oct;3(10):1763–1767. [PubMed] [Google Scholar]
- Andrews P. A., Jones J. A., Varki N. M., Howell S. B. Rapid emergence of acquired cis-diamminedichloroplatinum(II) resistance in an in vivo model of human ovarian carcinoma. Cancer Commun. 1990;2(2):93–100. doi: 10.3727/095535490820874641. [DOI] [PubMed] [Google Scholar]
- Anthoney D. A., McIlwrath A. J., Gallagher W. M., Edlin A. R., Brown R. Microsatellite instability, apoptosis, and loss of p53 function in drug-resistant tumor cells. Cancer Res. 1996 Mar 15;56(6):1374–1381. [PubMed] [Google Scholar]
- Ben-Yehuda D., Krichevsky S., Caspi O., Rund D., Polliack A., Abeliovich D., Zelig O., Yahalom V., Paltiel O., Or R. Microsatellite instability and p53 mutations in therapy-related leukemia suggest mutator phenotype. Blood. 1996 Dec 1;88(11):4296–4303. [PubMed] [Google Scholar]
- Bhattacharyya N. P., Skandalis A., Ganesh A., Groden J., Meuth M. Mutator phenotypes in human colorectal carcinoma cell lines. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6319–6323. doi: 10.1073/pnas.91.14.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. C., Umar A., Risinger J. I., Lipford J. R., Kane M., Yin S., Barrett J. C., Kolodner R. D., Kunkel T. A. Microsatellite instability, mismatch repair deficiency, and genetic defects in human cancer cell lines. Cancer Res. 1995 Dec 15;55(24):6063–6070. [PubMed] [Google Scholar]
- Chen A. Y., Liu L. F. DNA topoisomerases: essential enzymes and lethal targets. Annu Rev Pharmacol Toxicol. 1994;34:191–218. doi: 10.1146/annurev.pa.34.040194.001203. [DOI] [PubMed] [Google Scholar]
- Crameri A., Whitehorn E. A., Tate E., Stemmer W. P. Improved green fluorescent protein by molecular evolution using DNA shuffling. Nat Biotechnol. 1996 Mar;14(3):315–319. doi: 10.1038/nbt0396-315. [DOI] [PubMed] [Google Scholar]
- Drummond J. T., Anthoney A., Brown R., Modrich P. Cisplatin and adriamycin resistance are associated with MutLalpha and mismatch repair deficiency in an ovarian tumor cell line. J Biol Chem. 1996 Aug 16;271(33):19645–19648. doi: 10.1074/jbc.271.33.19645. [DOI] [PubMed] [Google Scholar]
- Duckett D. R., Drummond J. T., Murchie A. I., Reardon J. T., Sancar A., Lilley D. M., Modrich P. Human MutSalpha recognizes damaged DNA base pairs containing O6-methylguanine, O4-methylthymine, or the cisplatin-d(GpG) adduct. Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6443–6447. doi: 10.1073/pnas.93.13.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink D., Nebel S., Aebi S., Zheng H., Cenni B., Nehmé A., Christen R. D., Howell S. B. The role of DNA mismatch repair in platinum drug resistance. Cancer Res. 1996 Nov 1;56(21):4881–4886. [PubMed] [Google Scholar]
- Fink D., Zheng H., Nebel S., Norris P. S., Aebi S., Lin T. P., Nehmé A., Christen R. D., Haas M., MacLeod C. L. In vitro and in vivo resistance to cisplatin in cells that have lost DNA mismatch repair. Cancer Res. 1997 May 15;57(10):1841–1845. [PubMed] [Google Scholar]
- Fishel R., Kolodner R. D. Identification of mismatch repair genes and their role in the development of cancer. Curr Opin Genet Dev. 1995 Jun;5(3):382–395. doi: 10.1016/0959-437x(95)80055-7. [DOI] [PubMed] [Google Scholar]
- Griffin S., Branch P., Xu Y. Z., Karran P. DNA mismatch binding and incision at modified guanine bases by extracts of mammalian cells: implications for tolerance to DNA methylation damage. Biochemistry. 1994 Apr 26;33(16):4787–4793. doi: 10.1021/bi00182a006. [DOI] [PubMed] [Google Scholar]
- Hawn M. T., Umar A., Carethers J. M., Marra G., Kunkel T. A., Boland C. R., Koi M. Evidence for a connection between the mismatch repair system and the G2 cell cycle checkpoint. Cancer Res. 1995 Sep 1;55(17):3721–3725. [PubMed] [Google Scholar]
- Huang J., Papadopoulos N., McKinley A. J., Farrington S. M., Curtis L. J., Wyllie A. H., Zheng S., Willson J. K., Markowitz S. D., Morin P. APC mutations in colorectal tumors with mismatch repair deficiency. Proc Natl Acad Sci U S A. 1996 Aug 20;93(17):9049–9054. doi: 10.1073/pnas.93.17.9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kat A., Thilly W. G., Fang W. H., Longley M. J., Li G. M., Modrich P. An alkylation-tolerant, mutator human cell line is deficient in strand-specific mismatch repair. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6424–6428. doi: 10.1073/pnas.90.14.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koi M., Umar A., Chauhan D. P., Cherian S. P., Carethers J. M., Kunkel T. A., Boland C. R. Human chromosome 3 corrects mismatch repair deficiency and microsatellite instability and reduces N-methyl-N'-nitro-N-nitrosoguanidine tolerance in colon tumor cells with homozygous hMLH1 mutation. Cancer Res. 1994 Aug 15;54(16):4308–4312. [PubMed] [Google Scholar]
- Kolodner R. Biochemistry and genetics of eukaryotic mismatch repair. Genes Dev. 1996 Jun 15;10(12):1433–1442. doi: 10.1101/gad.10.12.1433. [DOI] [PubMed] [Google Scholar]
- Liu L., Markowitz S., Gerson S. L. Mismatch repair mutations override alkyltransferase in conferring resistance to temozolomide but not to 1,3-bis(2-chloroethyl)nitrosourea. Cancer Res. 1996 Dec 1;56(23):5375–5379. [PubMed] [Google Scholar]
- Loeb L. A. Microsatellite instability: marker of a mutator phenotype in cancer. Cancer Res. 1994 Oct 1;54(19):5059–5063. [PubMed] [Google Scholar]
- Markowitz S., Wang J., Myeroff L., Parsons R., Sun L., Lutterbaugh J., Fan R. S., Zborowska E., Kinzler K. W., Vogelstein B. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995 Jun 2;268(5215):1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- Mello J. A., Acharya S., Fishel R., Essigmann J. M. The mismatch-repair protein hMSH2 binds selectively to DNA adducts of the anticancer drug cisplatin. Chem Biol. 1996 Jul;3(7):579–589. doi: 10.1016/s1074-5521(96)90149-0. [DOI] [PubMed] [Google Scholar]
- Morin J. G., Hastings J. W. Energy transfer in a bioluminescent system. J Cell Physiol. 1971 Jun;77(3):313–318. doi: 10.1002/jcp.1040770305. [DOI] [PubMed] [Google Scholar]
- Naviaux R. K., Costanzi E., Haas M., Verma I. M. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J Virol. 1996 Aug;70(8):5701–5705. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehmé A., Baskaran R., Aebi S., Fink D., Nebel S., Cenni B., Wang J. Y., Howell S. B., Christen R. D. Differential induction of c-Jun NH2-terminal kinase and c-Abl kinase in DNA mismatch repair-proficient and -deficient cells exposed to cisplatin. Cancer Res. 1997 Aug 1;57(15):3253–3257. [PubMed] [Google Scholar]
- Palombo F., Gallinari P., Iaccarino I., Lettieri T., Hughes M., D'Arrigo A., Truong O., Hsuan J. J., Jiricny J. GTBP, a 160-kilodalton protein essential for mismatch-binding activity in human cells. Science. 1995 Jun 30;268(5219):1912–1914. doi: 10.1126/science.7604265. [DOI] [PubMed] [Google Scholar]
- Skipper H. E., Schabel F. M., Jr, Lloyd H. H. Experimental therapeutics and kinetics: selection and overgrowth of specifically and permanently drug-resistant tumor cells. Semin Hematol. 1978 Jul;15(3):207–219. [PubMed] [Google Scholar]
- Tomlinson I. P., Novelli M. R., Bodmer W. F. The mutation rate and cancer. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14800–14803. doi: 10.1073/pnas.93.25.14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wind N., Dekker M., Berns A., Radman M., te Riele H. Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination, and predisposition to cancer. Cell. 1995 Jul 28;82(2):321–330. doi: 10.1016/0092-8674(95)90319-4. [DOI] [PubMed] [Google Scholar]



