Abstract
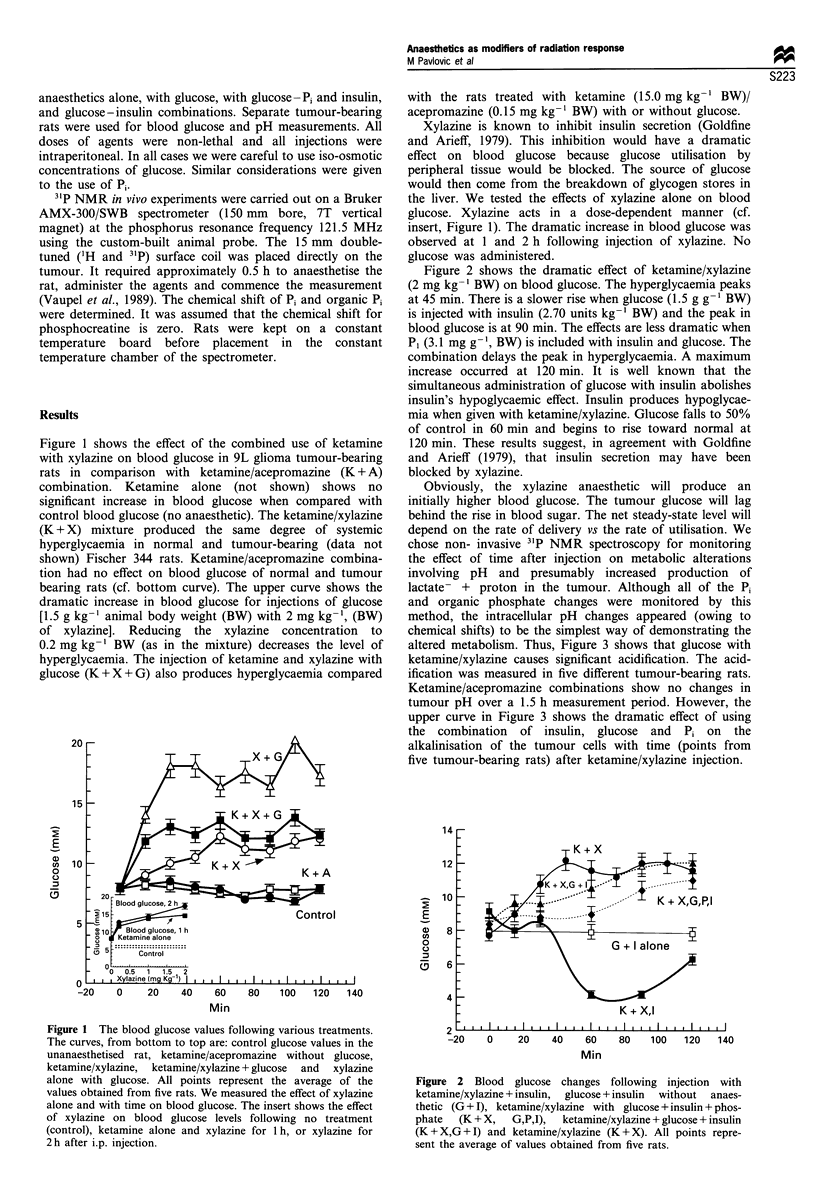
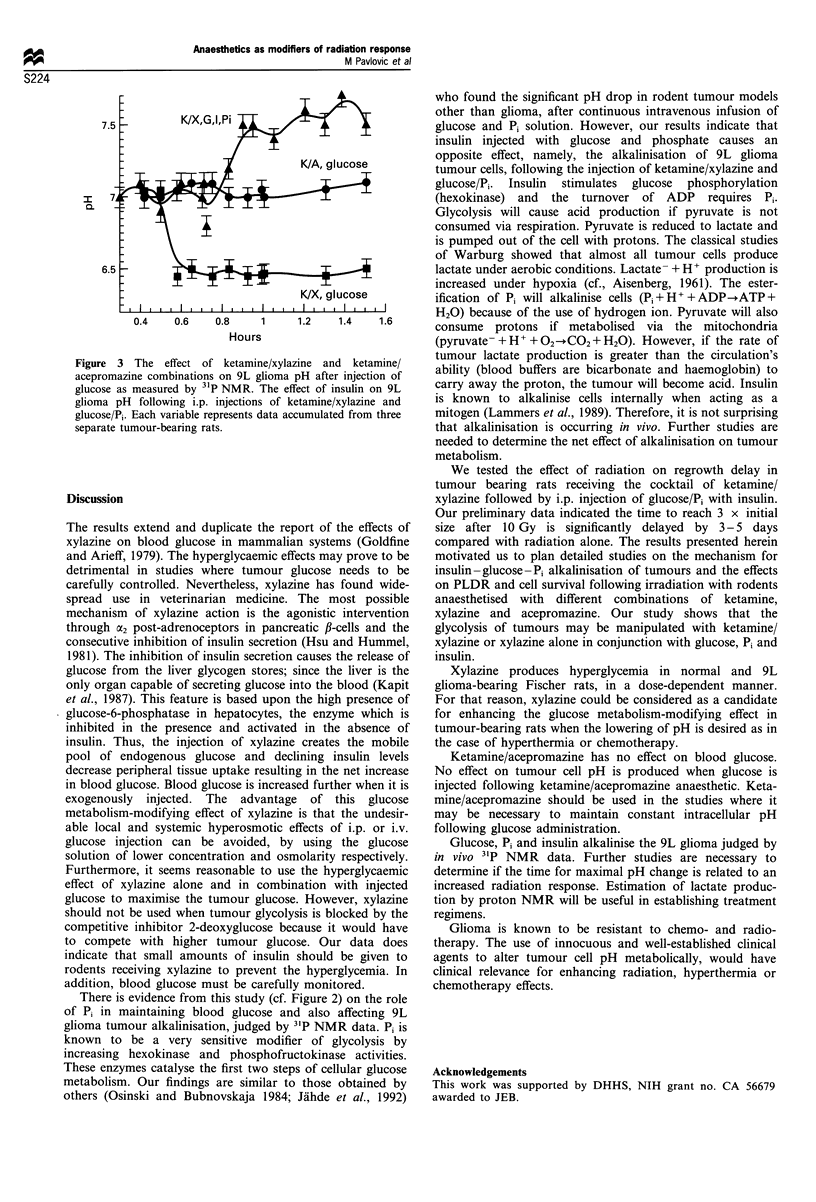
In the present study we demonstrate that the glycolysis of the tumour 9L glioma, in vivo, may be manipulated with ketamine/xylazine combinations of anaesthetics. Xylazine alone or in combination with ketamine causes hyperglycaemia which is enhanced by glucose injections. Intracellular tumour pH is acidified when glucose is administered with ketamine/xylazine. However, the combination of inorganic phosphate and insulin with ketamine/xylazine and glucose caused an alkaline shift in the tumour pH as measured by 31P NMR. The anaesthetic combination of ketamine/acepromazine did not produce alterations in blood glucose or in tumour pH status as detected by 31P NMR spectroscopy. These results demonstrate dramatic effects of ketamine/xylazine on the acidification or alkalinisation of the cells of 9L glioma. These altered metabolic states are of potential therapeutic importance. The choice of xylazine alone would be useful for chemotherapy and hyperthermia modalities, both known to be dependent upon glucose metabolism and resultant acidification.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biaglow J. E., Ferencz N., Jr, Friedell H. L. A metabolic control for the enhancement of radiation response. Am J Roentgenol Radium Ther Nucl Med. 1970 Feb;108(2):405–411. doi: 10.2214/ajr.108.2.405. [DOI] [PubMed] [Google Scholar]
- Biaglow J. E., Lavik P. S., Ferencz N., Jr Modification of radiation response through glucose-controlled respiration. Radiat Res. 1969 Sep;39(3):623–633. [PubMed] [Google Scholar]
- Biaglow J. E., Scroeder K., Durand R. E. The enhanced radiation response of in vitro tumor models to insulin. Int J Radiat Oncol Biol Phys. 1979 Sep;5(9):1669–1672. doi: 10.1016/0360-3016(79)90794-6. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Arieff A. I. Rapid inibition of basal and glucose-stimulated insulin release by xylazine. Endocrinology. 1979 Oct;105(4):920–922. doi: 10.1210/endo-105-4-920. [DOI] [PubMed] [Google Scholar]
- Hsu W. H., Hummel S. K. Xylazine-induced hyperglycemia in cattle: a possible involvement of alpha 2-adrenergic receptors regulating insulin release. Endocrinology. 1981 Sep;109(3):825–829. doi: 10.1210/endo-109-3-825. [DOI] [PubMed] [Google Scholar]
- Jähde E., Volk T., Atema A., Smets L. A., Glüsenkamp K. H., Rajewsky M. F. pH in human tumor xenografts and transplanted rat tumors: effect of insulin, inorganic phosphate, and m-iodobenzylguanidine. Cancer Res. 1992 Nov 15;52(22):6209–6215. [PubMed] [Google Scholar]
- Kallinowski F., Vaupel P. pH distributions in spontaneous and isotransplanted rat tumours. Br J Cancer. 1988 Sep;58(3):314–321. doi: 10.1038/bjc.1988.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers R., Gray A., Schlessinger J., Ullrich A. Differential signalling potential of insulin- and IGF-1-receptor cytoplasmic domains. EMBO J. 1989 May;8(5):1369–1375. doi: 10.1002/j.1460-2075.1989.tb03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinski S. P., Bubnovskaja L. N. Verstärkung der Tumorübersäuerung unter Bedingungen von künstlicher Hyperglykämie mit Hilfe anorganischen Phosphats. Arch Geschwulstforsch. 1984;54(6):463–469. [PubMed] [Google Scholar]
- Thistlethwaite A. J., Alexander G. A., Moylan D. J., 3rd, Leeper D. B. Modification of human tumor pH by elevation of blood glucose. Int J Radiat Oncol Biol Phys. 1987 Apr;13(4):603–610. doi: 10.1016/0360-3016(87)90078-2. [DOI] [PubMed] [Google Scholar]
- Varnes M. E., Dethlefsen L. A., Biaglow J. E. The effect of pH on potentially lethal damage recovery in A549 cells. Radiat Res. 1986 Oct;108(1):80–90. [PubMed] [Google Scholar]
- Vaupel P. W., Okunieff P. G. Role of hypovolemic hemoconcentration in dose-dependent flow decline observed in murine tumors after intraperitoneal administration of glucose or mannitol. Cancer Res. 1988 Dec 15;48(24 Pt 1):7102–7106. [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]


