Abstract
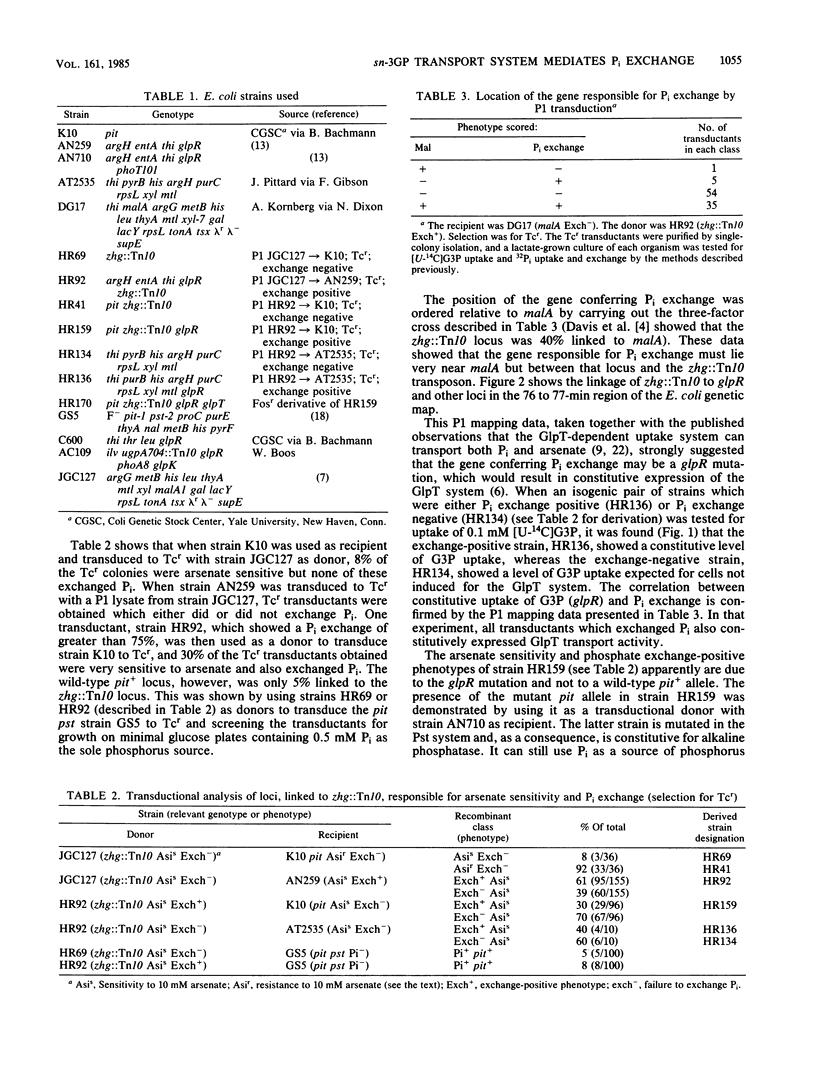
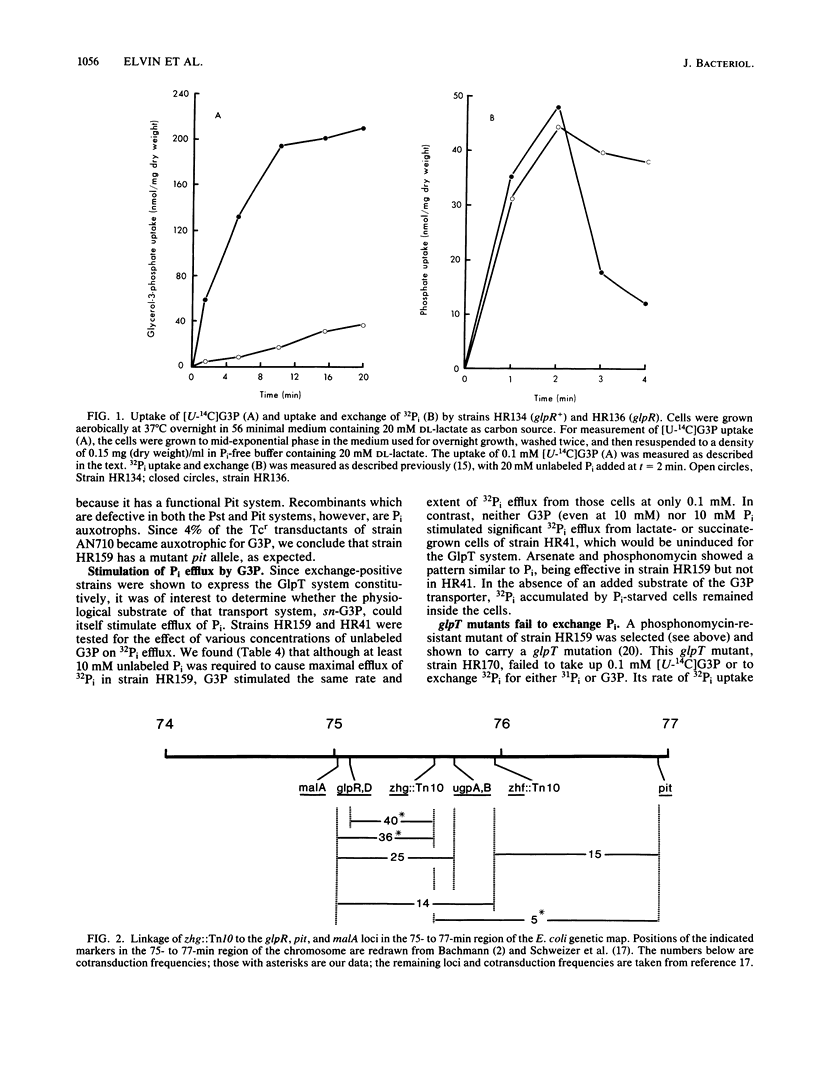
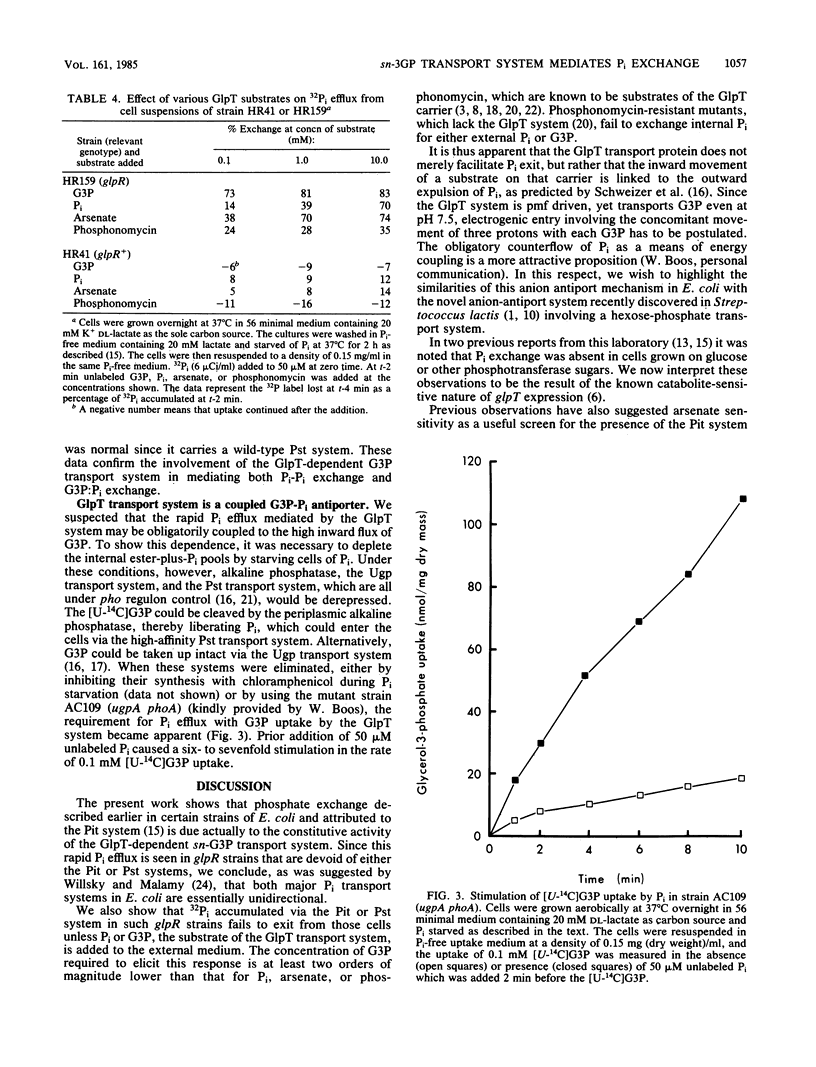
The GlpT system for sn-glycerol-3-phosphate transport in Escherichia coli is shown to catalyze a rapid efflux of Pi from the internal phosphate pools in response to externally added Pi or glycerol-3-phosphate. A glpR mutation, which results in constitutive expression of the GlpT system, is responsible for this rapid Pi efflux and the arsenate sensitivity of several laboratory strains, including the popular strain C600. Glucose and other phosphotransferase system sugars inhibit Pi efflux by repressing glpT expression.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambudkar S. V., Maloney P. C. Characterization of phosphate:hexose 6-phosphate antiport in membrane vesicles of Streptococcus lactis. J Biol Chem. 1984 Oct 25;259(20):12576–12585. [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. L., Malamy M. H. Arsenate resistant mutants of Escherichia coli and phosphate transport. Biochem Biophys Res Commun. 1970 Jul 27;40(2):496–503. doi: 10.1016/0006-291x(70)91036-3. [DOI] [PubMed] [Google Scholar]
- Davis N. K., Greer S., Jones-Mortimer M. C., Perham R. N. Isolation and mapping of glutathione reductase-negative mutants of Escherichia coli K12. J Gen Microbiol. 1982 Jul;128(7):1631–1634. doi: 10.1099/00221287-128-7-1631. [DOI] [PubMed] [Google Scholar]
- Ferguson W. J., Braunschweiger K. I., Braunschweiger W. R., Smith J. R., McCormick J. J., Wasmann C. C., Jarvis N. P., Bell D. H., Good N. E. Hydrogen ion buffers for biological research. Anal Biochem. 1980 May 15;104(2):300–310. doi: 10.1016/0003-2697(80)90079-2. [DOI] [PubMed] [Google Scholar]
- Freedberg W. B., Lin E. C. Three kinds of controls affecting the expression of the glp regulon in Escherichia coli. J Bacteriol. 1973 Sep;115(3):816–823. doi: 10.1128/jb.115.3.816-823.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassberg J., Meyer R. R., Kornberg A. Mutant single-strand binding protein of Escherichia coli: genetic and physiological characterization. J Bacteriol. 1979 Oct;140(1):14–19. doi: 10.1128/jb.140.1.14-19.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- Lin E. C. Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol. 1976;30:535–578. doi: 10.1146/annurev.mi.30.100176.002535. [DOI] [PubMed] [Google Scholar]
- Maloney P. C., Ambudkar S. V., Thomas J., Schiller L. Phosphate/hexose 6-phosphate antiport in Streptococcus lactis. J Bacteriol. 1984 Apr;158(1):238–245. doi: 10.1128/jb.158.1.238-245.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae A. S., Strickland K. P. Studies on phosphate transport in Escherichia coli. II. Effects of metabolic inhibitors and divalent cations. Biochim Biophys Acta. 1976 May 21;433(3):564–582. doi: 10.1016/0005-2736(76)90282-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., Gerdes R. G., Chegwidden K. Two systems for the uptake of phosphate in Escherichia coli. J Bacteriol. 1977 Aug;131(2):505–511. doi: 10.1128/jb.131.2.505-511.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H., Gerdes R. G., Harold F. M. Energy coupling to the transport of inorganic phosphate in Escherichia coli K12. Biochem J. 1979 Jan 15;178(1):133–137. doi: 10.1042/bj1780133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H., Russell L. M., Jacomb P. A., Chegwidden K. Phosphate exchange in the pit transport system in Escherichia coli. J Bacteriol. 1982 Jan;149(1):123–130. doi: 10.1128/jb.149.1.123-130.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer H., Argast M., Boos W. Characteristics of a binding protein-dependent transport system for sn-glycerol-3-phosphate in Escherichia coli that is part of the pho regulon. J Bacteriol. 1982 Jun;150(3):1154–1163. doi: 10.1128/jb.150.3.1154-1163.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer H., Grussenmeyer T., Boos W. Mapping of two ugp genes coding for the pho regulon-dependent sn-glycerol-3-phosphate transport system of Escherichia coli. J Bacteriol. 1982 Jun;150(3):1164–1171. doi: 10.1128/jb.150.3.1164-1171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague G. F., Jr, Bell R. M., Cronan J. E., Jr A mutant of Escherichia coli auxotrophic for organic phosphates: evidence for two defects in inorganic phosphate transport. Mol Gen Genet. 1975 Dec 30;143(1):71–77. doi: 10.1007/BF00269422. [DOI] [PubMed] [Google Scholar]
- Surin B. P., Jans D. A., Fimmel A. L., Shaw D. C., Cox G. B., Rosenberg H. Structural gene for the phosphate-repressible phosphate-binding protein of Escherichia coli has its own promoter: complete nucleotide sequence of the phoS gene. J Bacteriol. 1984 Mar;157(3):772–778. doi: 10.1128/jb.157.3.772-778.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateswaran P. S., Wu H. C. Isolation and characterization of a phosphonomycin-resistant mutant of Escherichia coli K-12. J Bacteriol. 1972 Jun;110(3):935–944. doi: 10.1128/jb.110.3.935-944.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L. Overlapping and separate controls on the phosphate regulon in Escherichia coli K12. J Mol Biol. 1983 May 25;166(3):283–308. doi: 10.1016/s0022-2836(83)80086-2. [DOI] [PubMed] [Google Scholar]
- Willsky G. R., Bennett R. L., Malamy M. H. Inorganic phosphate transport in Escherichia coli: involvement of two genes which play a role in alkaline phosphatase regulation. J Bacteriol. 1973 Feb;113(2):529–539. doi: 10.1128/jb.113.2.529-539.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R., Malamy M. H. Characterization of two genetically separable inorganic phosphate transport systems in Escherichia coli. J Bacteriol. 1980 Oct;144(1):356–365. doi: 10.1128/jb.144.1.356-365.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R., Malamy M. H. Control of the synthesis of alkaline phosphatase and the phosphate-binding protein in Escherichia coli. J Bacteriol. 1976 Jul;127(1):595–609. doi: 10.1128/jb.127.1.595-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R., Malamy M. H. Effect of arsenate on inorganic phosphate transport in Escherichia coli. J Bacteriol. 1980 Oct;144(1):366–374. doi: 10.1128/jb.144.1.366-374.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



