Abstract
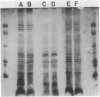
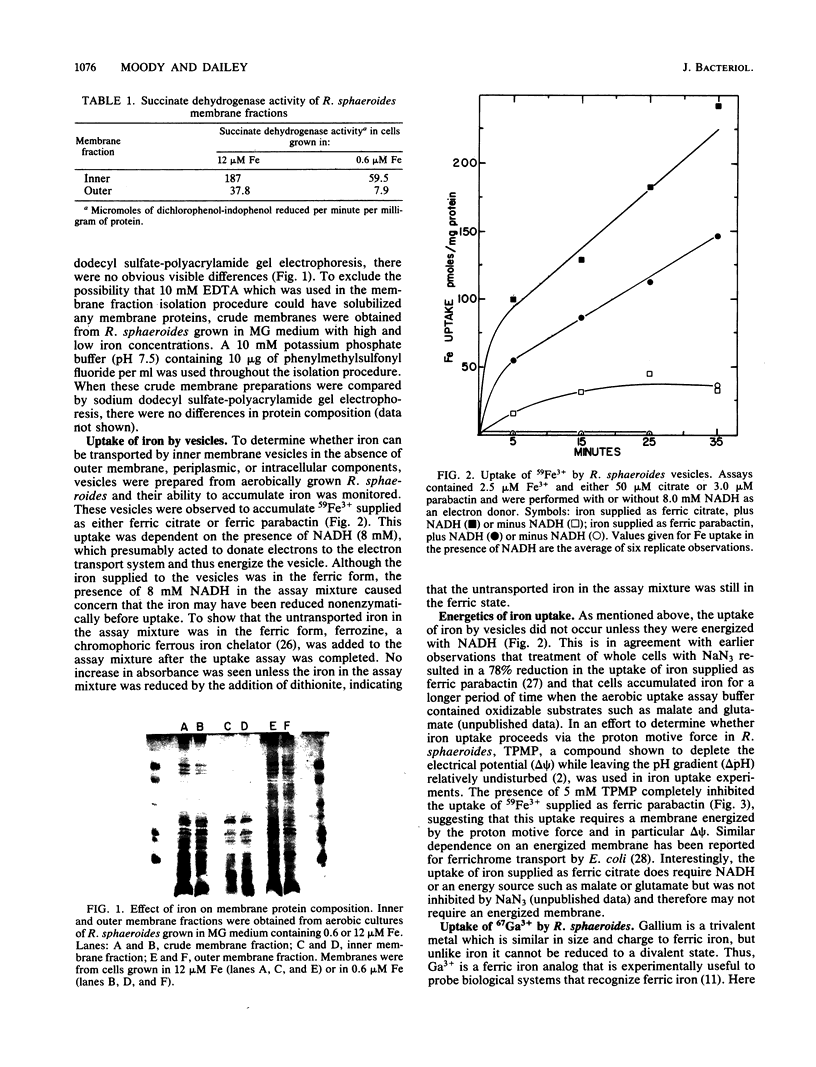
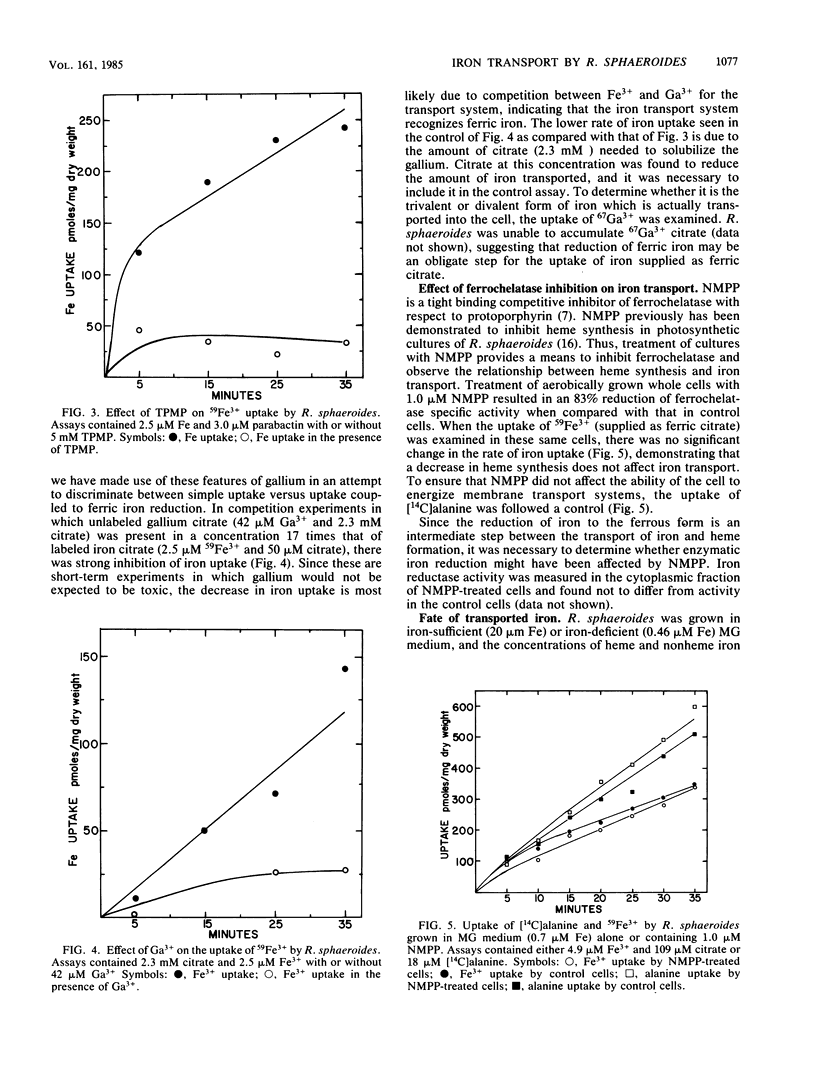
The uptake of iron supplied as ferric citrate or ferric parabactin was examined in aerobically grown whole cells and vesicles of Rhodopseudomonas sphaeroides. Inner and outer membrane fractions from R. sphaeroides contained no membrane proteins which were inducible by growth in low-iron medium. Vesicles composed of the inner membrane and devoid of outer membrane and periplasmic proteins were able to transport iron supplied as ferric citrate and ferric parabactin. This uptake required the presence of NADH. When the electrical component of the proton motive force was depleted in whole cells, the uptake of iron supplied as ferric parabactin was completely inhibited. The uptake of iron supplied as ferric citrate was inhibited by gallium citrate; however, Ga3+ was not transported. The relationship between iron uptake and heme synthesis was examined by treating whole cells with N-methylprotoporphyrin which inhibits ferrochelatase, the enzyme which inserts ferrous iron into protoporphyrin to form heme. This treatment reduced ferrochelatase activity by 82% but had no effect on iron uptake, indicating that iron uptake and heme synthesis are not directly coupled. The fate of transported iron was investigated by measuring intracellular concentrations of heme and nonheme iron. It was determined that newly transported iron exists primarily as nonheme iron.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boogerd F. C., Van Verseveld H. W., Stouthamer A. H. Respiration-driven proton translocation with nitrite and nitrous oxide in Paracoccus denitrificans. Biochim Biophys Acta. 1981 Dec 14;638(2):181–191. doi: 10.1016/0005-2728(81)90226-7. [DOI] [PubMed] [Google Scholar]
- Braun V., Hancock R. E., Hantke K., Hartmann A. Functional organization of the outer membrane of escherichia coli: phage and colicin receptors as components of iron uptake systems. J Supramol Struct. 1976;5(1):37–58. doi: 10.1002/jss.400050105. [DOI] [PubMed] [Google Scholar]
- Dailey H. A., Fleming J. E. Bovine ferrochelatase. Kinetic analysis of inhibition by N-methylprotoporphyrin, manganese, and heme. J Biol Chem. 1983 Oct 10;258(19):11453–11459. [PubMed] [Google Scholar]
- Dailey H. A., Jr Membrane-bound respiratory of Spirillum itersonii. J Bacteriol. 1976 Sep;127(3):1286–1291. doi: 10.1128/jb.127.3.1286-1291.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey H. A. Purification and characterization of membrane-bound ferrochelatase from Rhodopseudomonas sphaeroides. J Biol Chem. 1982 Dec 25;257(24):14714–14718. [PubMed] [Google Scholar]
- Ding D. H., Kaplan S. Separation of inner and outer membranes of Rhodopseudomonas spheroides. Prep Biochem. 1976;6(1):61–79. doi: 10.1080/00327487608061599. [DOI] [PubMed] [Google Scholar]
- Ecker D. J., Lancaster J. R., Jr, Emery T. Siderophore iron transport followed by electron paramagnetic resonance spectroscopy. J Biol Chem. 1982 Aug 10;257(15):8623–8626. [PubMed] [Google Scholar]
- Emery T., Hoffer P. B. Siderophore-mediated mechanism of gallium uptake demonstrated in the microorganism Ustilago sphaerogena. J Nucl Med. 1980 Oct;21(10):935–939. [PubMed] [Google Scholar]
- Granick S., Beale S. I. Hemes, chlorophylls, and related compounds: biosynthesis and metabolic regulation. Adv Enzymol Relat Areas Mol Biol. 1978;46:33–203. doi: 10.1002/9780470122914.ch2. [DOI] [PubMed] [Google Scholar]
- Hancock R. E. Alterations in outer membrane permeability. Annu Rev Microbiol. 1984;38:237–264. doi: 10.1146/annurev.mi.38.100184.001321. [DOI] [PubMed] [Google Scholar]
- Hellingwerf K. J., Michels P. A., Dorpema J. W., Konings W. N. Transport of amino acids in membrane vesicles of Rhodopseudomonas spheroides energized by respiratory and cyclic electron flow. Eur J Biochem. 1975 Jul 1;55(2):397–406. doi: 10.1111/j.1432-1033.1975.tb02175.x. [DOI] [PubMed] [Google Scholar]
- Houghton J. D., Honeybourne C. L., Smith K. M., Tabba H. D., Jones O. T. The use of N-methylprotoporphyrin dimethyl ester to inhibit ferrochelatase in Rhodopseudomonas sphaeroides and its effect in promoting biosynthesis of magnesium tetrapyrroles. Biochem J. 1982 Nov 15;208(2):479–486. doi: 10.1042/bj2080479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein S., Hantke K., Braun V. Citrate-dependent iron transport system in Escherichia coli K-12. Eur J Biochem. 1981 Jul;117(2):431–437. doi: 10.1111/j.1432-1033.1981.tb06357.x. [DOI] [PubMed] [Google Scholar]
- Imai K., Asano A., Sato R. Oxidative phosphorylation in Micrococcus denitrificans. V. Effects of iron deficiency on respiratory components and oxidative phosphorylation. J Biochem. 1968 Feb;63(2):219–225. doi: 10.1093/oxfordjournals.jbchem.a128764. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. Adaptation to form bacteriochlorophyll in Rhodopseudomonas spheroides: changes in activity of enzymes concerned in pyrrole synthesis. Biochem J. 1959 Jul;72:508–518. doi: 10.1042/bj0720508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lascelles J. The regulation of haem and chlorophyll synthesis. Biochem Soc Symp. 1968;28:49–59. [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Moody M. D., Dailey H. A. Aerobic ferrisiderophore reductase assay and activity stain for native polyacrylamide gels. Anal Biochem. 1983 Oct 1;134(1):235–239. doi: 10.1016/0003-2697(83)90290-7. [DOI] [PubMed] [Google Scholar]
- Moody M. D., Dailey H. A. Siderophore utilization and iron uptake by Rhodopseudomonas sphaeroides. Arch Biochem Biophys. 1984 Oct;234(1):178–186. doi: 10.1016/0003-9861(84)90339-4. [DOI] [PubMed] [Google Scholar]
- Negrin R. S., Neilands J. B. Ferrichrome transport in inner membrane vesicles of Escherichia coli K12. J Biol Chem. 1978 Apr 10;253(7):2339–2342. [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- O'Brien I. G., Gibson F. The structure of enterochelin and related 2,3-dihydroxy-N-benzoylserine conjugates from Escherichia coli. Biochim Biophys Acta. 1970 Aug 14;215(2):393–402. doi: 10.1016/0304-4165(70)90038-3. [DOI] [PubMed] [Google Scholar]
- Reiss-Husson F., De Klerk H., Jolchine G., Jauneau E., Kamen M. D. Some effects of iron deficiency on Rhodopseudomonas spheroides strain Y. Biochim Biophys Acta. 1971 Apr 6;234(1):73–82. doi: 10.1016/0005-2728(71)90132-0. [DOI] [PubMed] [Google Scholar]
- Sinclair P. R., White D. C. Effect of nitrate, fumarate, and oxygen on the formation of the membrane-bound electron transport system of Haemophilus parainfluenzae. J Bacteriol. 1970 Feb;101(2):365–372. doi: 10.1128/jb.101.2.365-372.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T. P. Determination of the activity of succinate, NADH, choline, and alpha-glycerophosphate dehydrogenases. Methods Biochem Anal. 1974;22:123–175. doi: 10.1002/9780470110423.ch3. [DOI] [PubMed] [Google Scholar]
- Tait G. H. The identification and biosynthesis of siderochromes formed by Micrococcus denitrificans. Biochem J. 1975 Jan;146(1):191–204. doi: 10.1042/bj1460191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckesser J., Zalman L. S., Nikaido H. Porin from Rhodopseudomonas sphaeroides. J Bacteriol. 1984 Jul;159(1):199–205. doi: 10.1128/jb.159.1.199-205.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]