Abstract
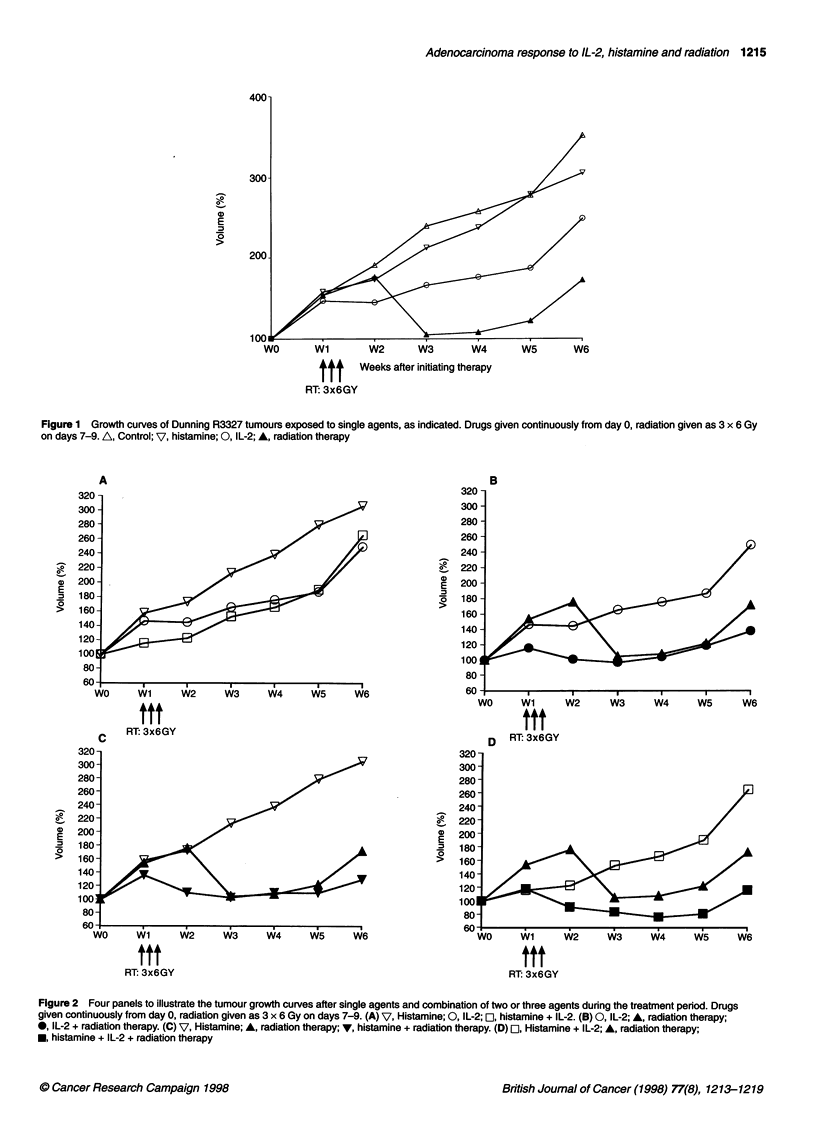
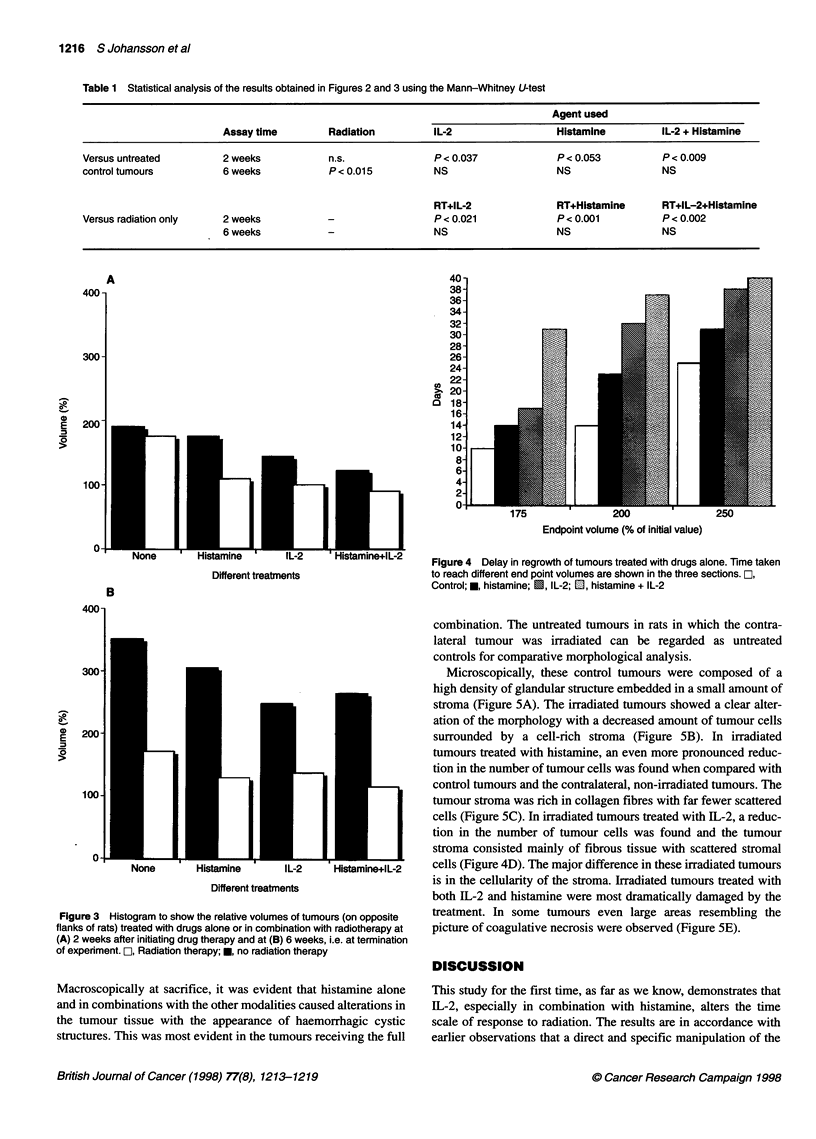
A syngeneic, androgen-sensitive Dunning R3327 rat prostatic adenocarcinoma was transplanted bilaterally in the flanks of male Copenhagen Fisher rats. Approximately 3 months after implantation, when the tumours had a median volume of 150 mm3, one group of rats was treated with histamine alone (4 mg kg(-1) subcutaneously on week days), another group with human recombinant interleukin 2 (IL-2) alone (425 IU kg(-1) continuous infusion) and a third group with both histamine and IL-2 during 6 weeks. Tumours on one flank were irradiated (6 Gy once daily for 3 days to a total dose of 18 Gy) beginning 1 week after the onset of treatment with histamine and/or IL-2. The contralateral tumour served as the intra-animal control. The tumour volumes were determined weekly. The growth curves showed that all three drug treatments were effective in delaying growth, but when used individually did not cause tumour shrinkage. Radiation was the most effective single agent, but when used alone the shrinkage did not occur until 2 weeks after irradiation. When combined with the drugs, more rapid and extensive growth delay and/or shrinkage was seen. The growth curves showed clear differences between the different treatments. The combination of the three agents was the most effective of all. The most striking difference between radiation alone and radiation plus biotherapy was the time at which a tumour response was detectable. Thus, active biotherapy alone and especially in a combination with histamine and radiotherapy warrants further investigation as a potential therapeutic approach to prostate cancer.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asea A., Hermodsson S., Hellstrand K. Histaminergic regulation of natural killer cell-mediated clearance of tumour cells in mice. Scand J Immunol. 1996 Jan;43(1):9–15. doi: 10.1046/j.1365-3083.1996.d01-14.x. [DOI] [PubMed] [Google Scholar]
- Atzpodien J., Lopez Hänninen E., Kirchner H., Bodenstein H., Pfreundschuh M., Rebmann U., Metzner B., Illiger H. J., Jakse G., Niesel T. Multiinstitutional home-therapy trial of recombinant human interleukin-2 and interferon alfa-2 in progressive metastatic renal cell carcinoma. J Clin Oncol. 1995 Feb;13(2):497–501. doi: 10.1200/JCO.1995.13.2.497. [DOI] [PubMed] [Google Scholar]
- Brune M., Hellstrand K. Remission maintenance therapy with histamine and interleukin-2 in acute myelogenous leukaemia. Br J Haematol. 1996 Mar;92(3):620–626. doi: 10.1046/j.1365-2141.1996.00389.x. [DOI] [PubMed] [Google Scholar]
- Burtin C., Ponvert C., Fray A., Scheinmann P., Lespinats G., Loridon B., Canu P., Paupe J. Inverse correlation between tumor incidence and tissue histamine levels in W/WV, WV/+, and +/+ mice. J Natl Cancer Inst. 1985 Mar;74(3):671–674. [PubMed] [Google Scholar]
- Caligiuri M. A., Murray C., Robertson M. J., Wang E., Cochran K., Cameron C., Schow P., Ross M. E., Klumpp T. R., Soiffer R. J. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J Clin Invest. 1993 Jan;91(1):123–132. doi: 10.1172/JCI116161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denekamp J. Vascular attack as a therapeutic strategy for cancer. Cancer Metastasis Rev. 1990 Nov;9(3):267–282. doi: 10.1007/BF00046365. [DOI] [PubMed] [Google Scholar]
- Hellstrand K., Asea A., Dahlgren C., Hermodsson S. Histaminergic regulation of NK cells. Role of monocyte-derived reactive oxygen metabolites. J Immunol. 1994 Dec 1;153(11):4940–4947. [PubMed] [Google Scholar]
- Hellstrand K., Hermodsson S. Synergistic activation of human natural killer cell cytotoxicity by histamine and interleukin-2. Int Arch Allergy Appl Immunol. 1990;92(4):379–389. doi: 10.1159/000235169. [DOI] [PubMed] [Google Scholar]
- Hellstrand K., Naredi P., Lindner P., Lundholm K., Rudenstam C. M., Hermodsson S., Asztély M., Hafström L. Histamine in immunotherapy of advanced melanoma: a pilot study. Cancer Immunol Immunother. 1994 Dec;39(6):416–419. doi: 10.1007/BF01534430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson R., Widmark A., Bergh A., Damber J. E. Interleukin-2-induced growth inhibition of prostatic adenocarcinoma (Dunning R3327) in rats. Urol Res. 1992;20(3):189–191. doi: 10.1007/BF00299715. [DOI] [PubMed] [Google Scholar]
- Isaacs J. T. Development and characteristics of the available animal model systems for the study of prostatic cancer. Prog Clin Biol Res. 1987;239:513–576. [PubMed] [Google Scholar]
- Lahat N., Alexander B., Levin D. R., Moskovitz B. The relationship between clinical stage, natural killer activity and related immunological parameters in adenocarcinoma of the prostate. Cancer Immunol Immunother. 1989;28(3):208–212. doi: 10.1007/BF00204990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landström M., Funa K. Apoptosis in rat prostatic adenocarcinoma is associated with rapid infiltration of cytotoxic T-cells and activated macrophages. Int J Cancer. 1997 May 2;71(3):451–455. doi: 10.1002/(sici)1097-0215(19970502)71:3<451::aid-ijc24>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Mador D., Ritchie B., Meeker B., Moore R., Elliott F. G., McPhee M. S., Chapman J. D., Lakey W. H. Response of the Dunning R3327H prostatic adenocarcinoma to radiation and various chemotherapeutic drugs. Cancer Treat Rep. 1982 Oct;66(10):1837–1843. [PubMed] [Google Scholar]
- Meikle A. W., Smith J. A., Stringham J. D. Production, clearance, and metabolism of testosterone in men with prostatic cancer. Prostate. 1987;10(1):25–31. doi: 10.1002/pros.2990100106. [DOI] [PubMed] [Google Scholar]
- Meyer JS, He W. Cell Proliferation Measurements by Bromodeoxyuridine or Thymidine Incorporation: Clinical Correlates. Semin Radiat Oncol. 1993 Apr;3(2):126–134. doi: 10.1054/SRAO00300126. [DOI] [PubMed] [Google Scholar]
- Moody D. B., Robinson J. C., Ewing C. M., Lazenby A. J., Isaacs W. B. Interleukin-2 transfected prostate cancer cells generate a local antitumor effect in vivo. Prostate. 1994 May;24(5):244–251. doi: 10.1002/pros.2990240505. [DOI] [PubMed] [Google Scholar]
- Peri G., Chiaffarino F., Bernasconi S., Padura I. M., Mantovani A. Cytotoxicity of activated monocytes on endothelial cells. J Immunol. 1990 Feb 15;144(4):1444–1448. [PubMed] [Google Scholar]
- Reizenstein P., Ogier C., Blomgren H., Petrini B., Wasserman J. Cells responsible for tumor surveillance in man: effects of radiotherapy, chemotherapy, and biologic response modifiers. Adv Immun Cancer Ther. 1985;1:1–28. doi: 10.1007/978-1-4612-5068-5_1. [DOI] [PubMed] [Google Scholar]
- Smolev J. K., Coffey D. S., Scott W. W. Experimental models for the study of prostatic adenocarcinoma. J Urol. 1977 Jul;118(1 Pt 2):216–220. doi: 10.1016/s0022-5347(17)57949-5. [DOI] [PubMed] [Google Scholar]
- Thorndyke C., Meeker B. E., Thomas G., Lakey W. H., McPhee M. S., Chapman J. D. The radiation sensitivities of R3327-H and R3327-AT rat prostate adenocarcinomas. J Urol. 1985 Jul;134(1):191–198. doi: 10.1016/s0022-5347(17)47055-8. [DOI] [PubMed] [Google Scholar]
- Van Loveren H., Den Otter W., Meade R., Terheggen P. M., Askenase P. W. A role for mast cells and the vasoactive amine serotonin in T cell-dependent immunity to tumors. J Immunol. 1985 Feb;134(2):1292–1299. [PubMed] [Google Scholar]



