Abstract
DT-diaphorase is a two-electron-reducing enzyme that is an important activator of bioreductive anti-tumour agents, such as mitomycin C (MMC) and EO9, and is inducible by many compounds, including 1,2-dithiole-3-thiones (D3Ts). We showed previously that D3T selectively increased DT-diaphorase activity in mouse lymphoma cells compared with normal mouse marrow cells, and also increased MMC or EO9 cytotoxic activity in the lymphoma cells with only minor effects in the marrow cells. In this study, we found that D3T significantly increased DT-diaphorase activity in 28 of 38 human tumour cell lines representing ten tissue types with no obvious relationships between the tumour type, or the base level of DT-diaphorase activity, and the ability of D3T to increase the enzyme activity. Induction of DT-diaphorase activity in human tumour cell lines by 12 D3T analogues varied markedly with the D3T structure. D3T also increased DT-diaphorase activity in normal human bone marrow and kidney cells but the increases were small in these cells. In addition, D3T increased the level of enzyme activity in normal human lung cells. Pretreatment of human tumour cells with D3T analogues significantly increased the cytotoxic activity of MMC or EO9 in these cells, and the level of enhancement of anti-tumour activity paralleled the level of DT-diaphorase induction. In contrast, D3T did not effect the toxicity of EO9 in normal kidney cells. These results demonstrate that D3T analogues can increase DT-diaphorase activity in a wide variety of human tumour cells and that this effect can enhance the anti-tumour activity of the bioreductive agents MMC and EO9.
Full text
PDF

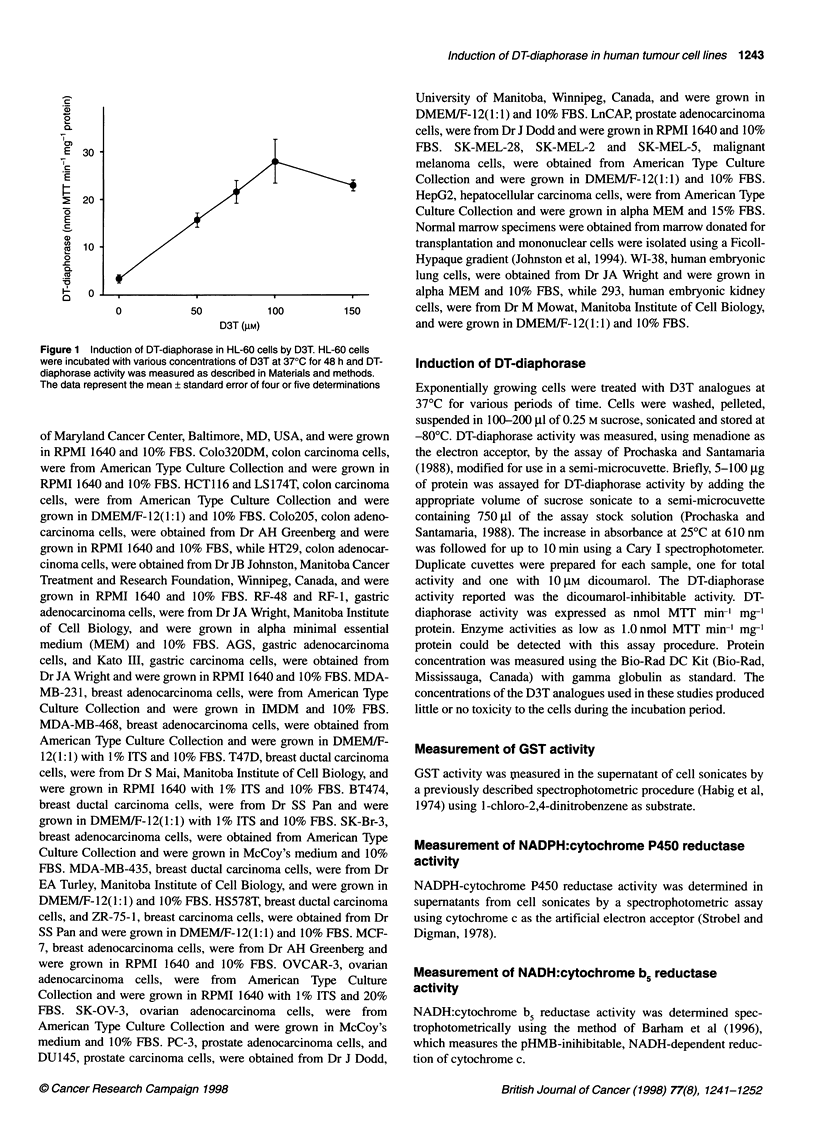
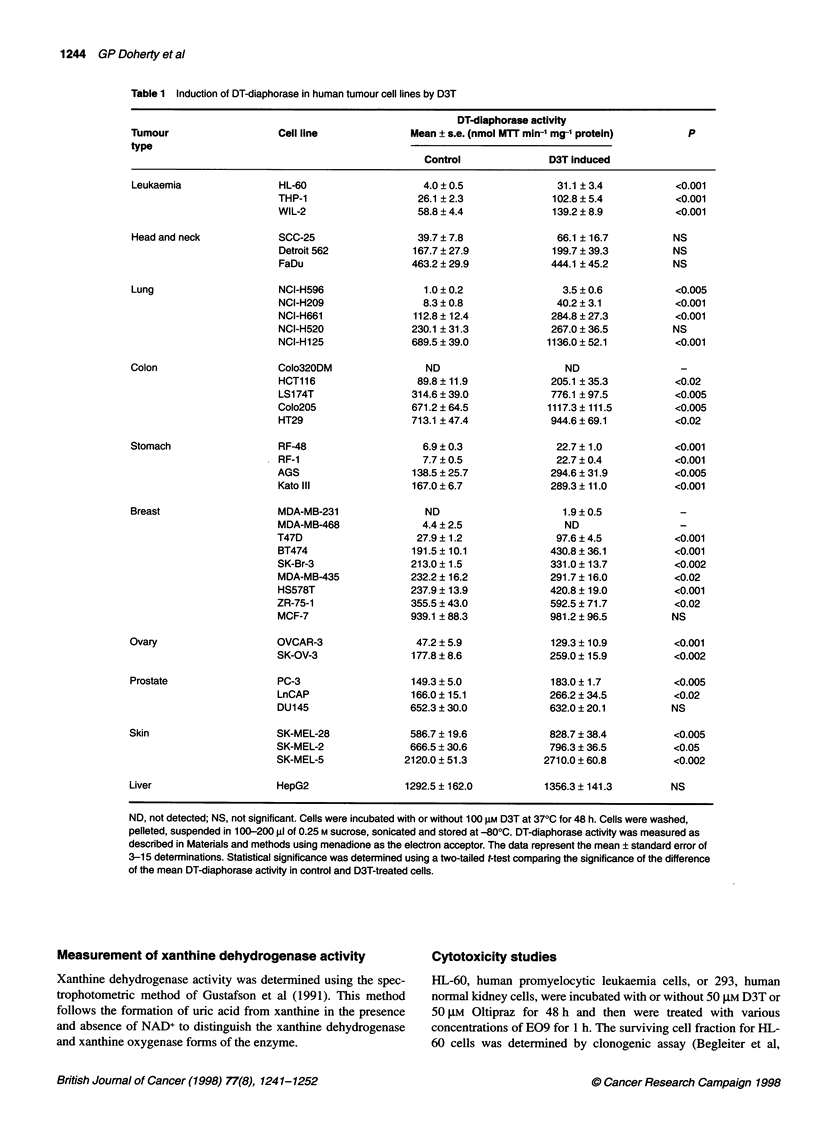
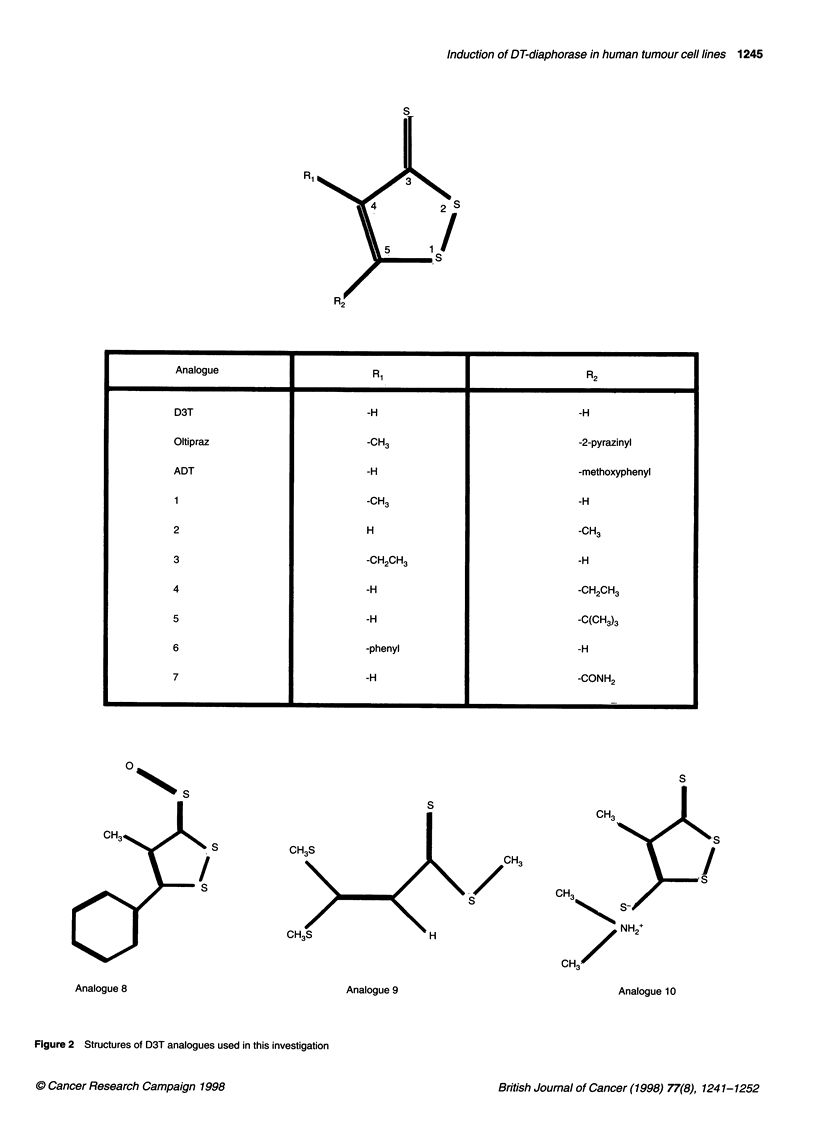
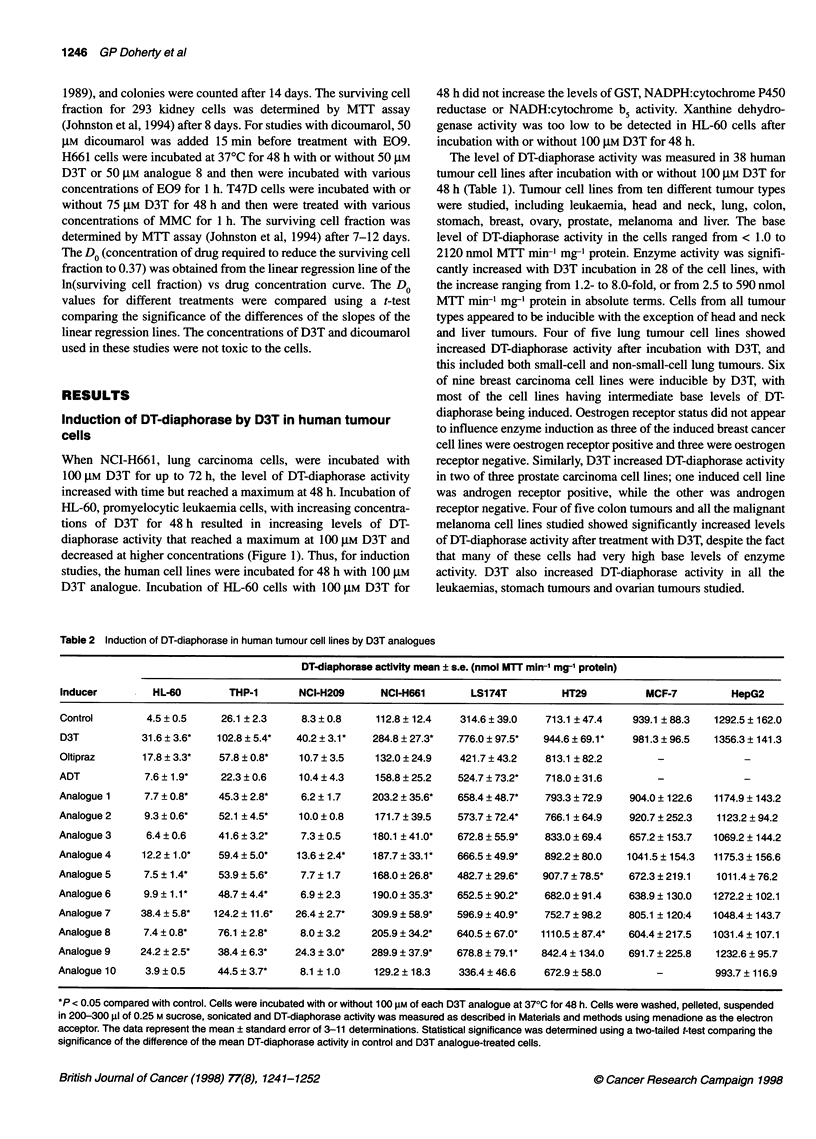
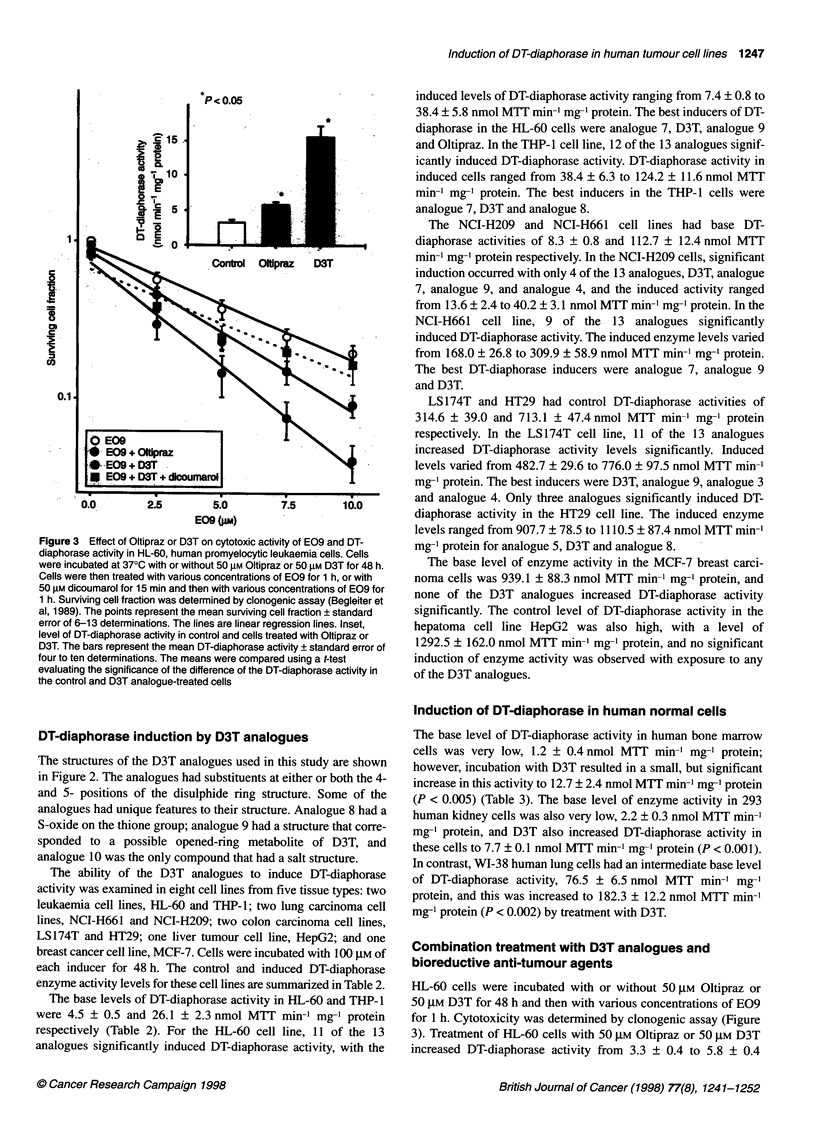
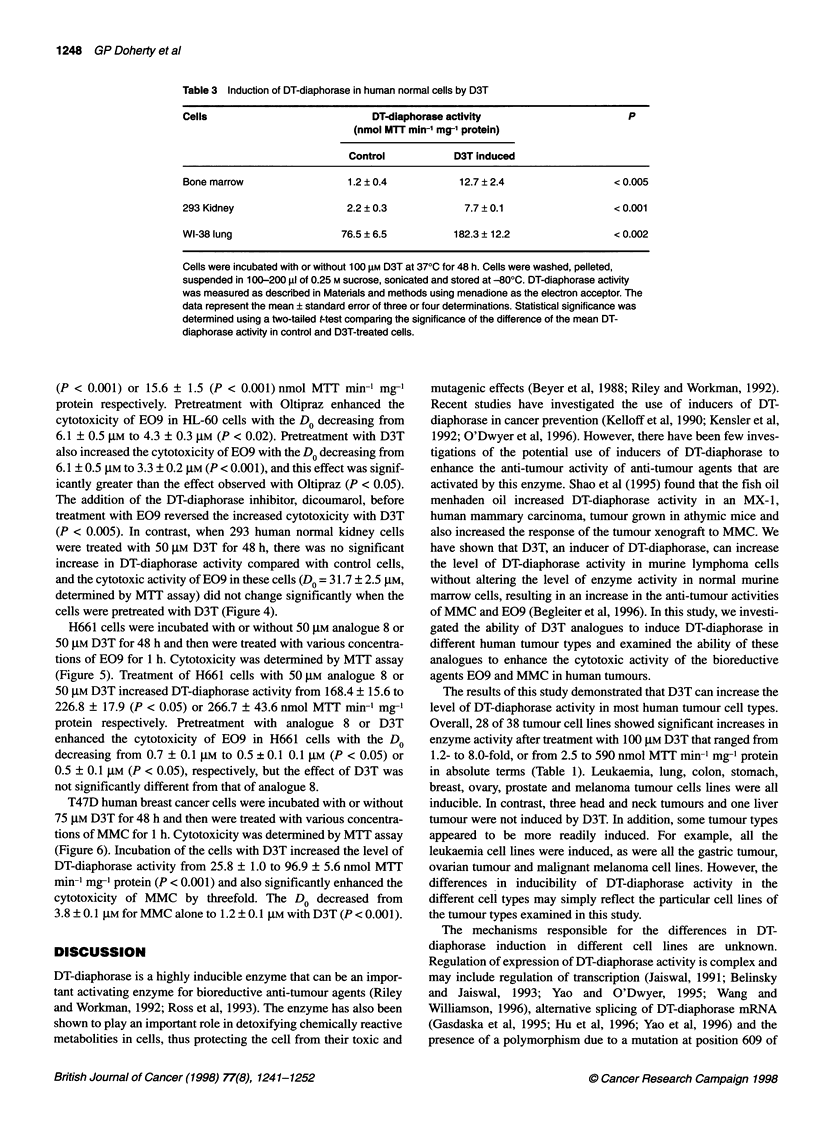
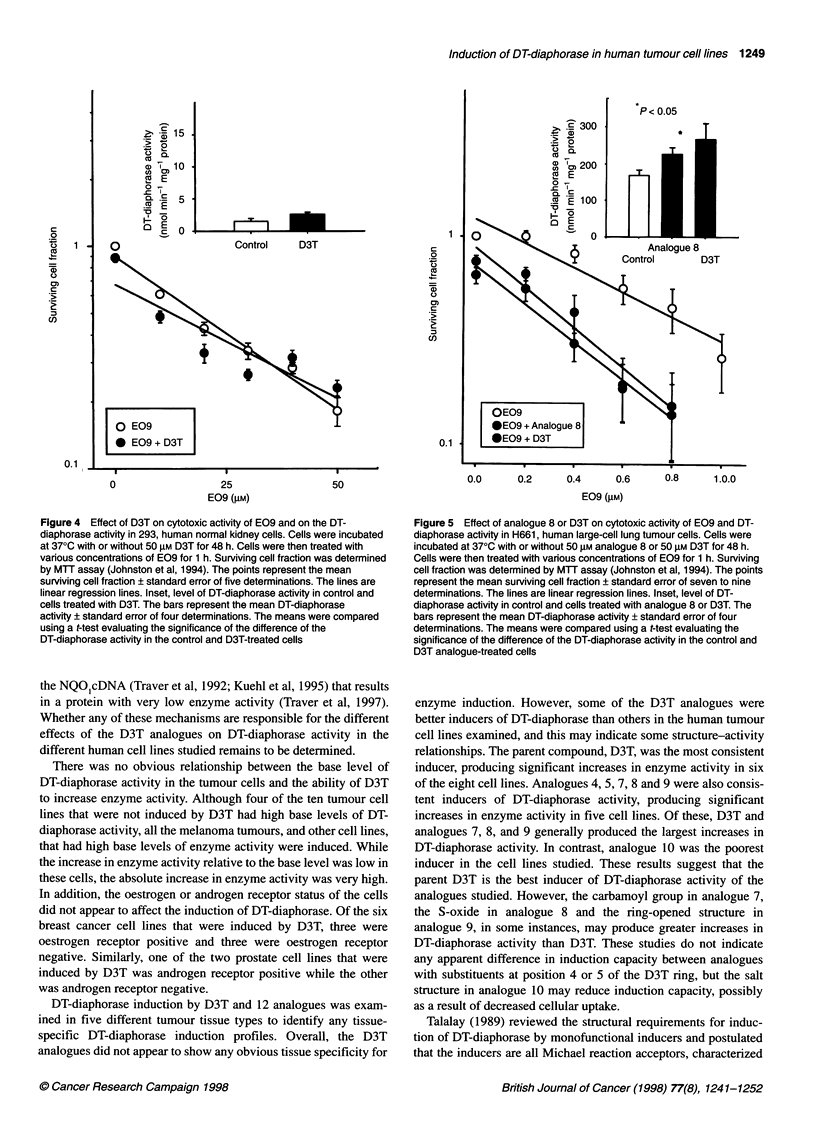
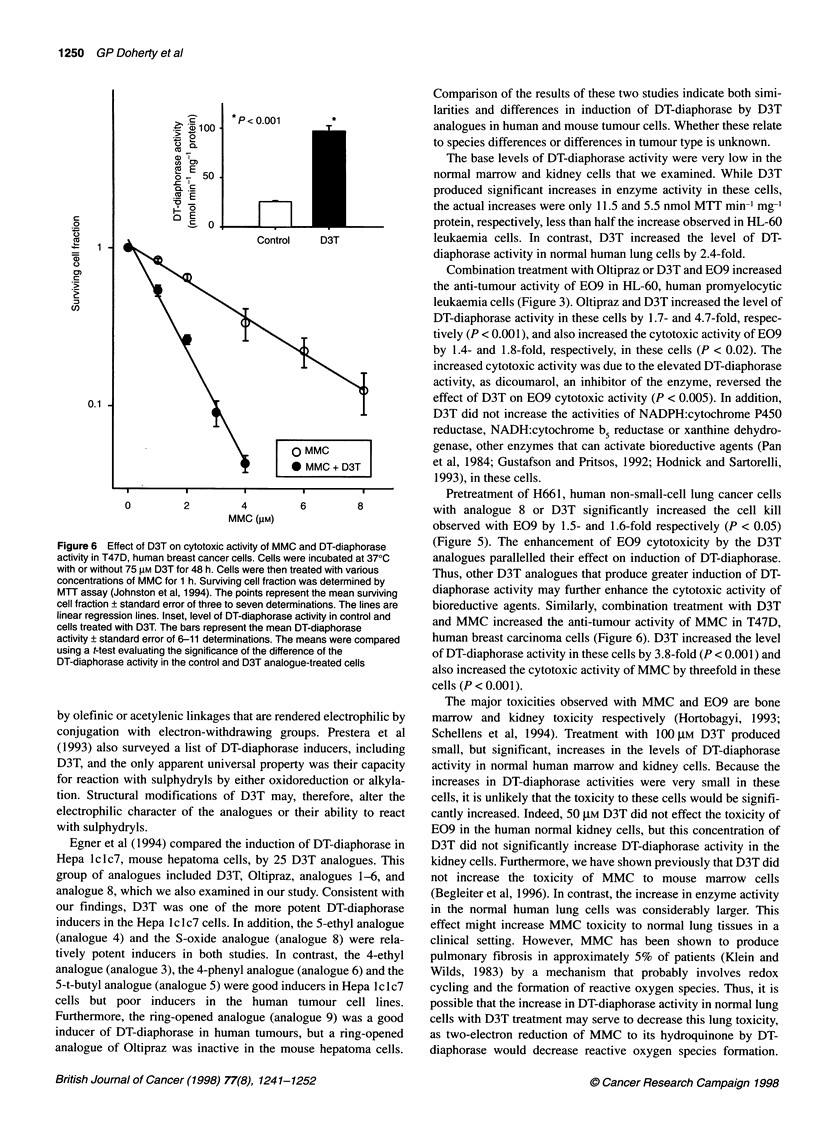


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atallah A. S., Landolph J. R., Ernster L., Hochstein P. DT-diaphorase activity and the cytotoxicity of quinones in C3H/10T1/2 mouse embryo cells. Biochem Pharmacol. 1988 Jun 15;37(12):2451–2459. doi: 10.1016/0006-2952(88)90373-5. [DOI] [PubMed] [Google Scholar]
- Barham H. M., Inglis R., Chinje E. C., Stratford I. J. Development and validation of a spectrophotometric assay for measuring the activity of NADH: cytochrome b5 reductase in human tumour cells. Br J Cancer. 1996 Oct;74(8):1188–1193. doi: 10.1038/bjc.1996.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall H. D., Liu Y., Siegel D., Bolton E. M., Gibson N. W., Ross D. Role of NAD(P)H:quinone oxidoreductase (DT-diaphorase) in cytotoxicity and induction of DNA damage by streptonigrin. Biochem Pharmacol. 1996 Mar 8;51(5):645–652. doi: 10.1016/s0006-2952(95)00223-5. [DOI] [PubMed] [Google Scholar]
- Begleiter A., Leith M. K. Activity of quinone alkylating agents in quinone-resistant cells. Cancer Res. 1990 May 15;50(10):2872–2876. [PubMed] [Google Scholar]
- Begleiter A., Leith M. K., Curphey T. J. Induction of DT-diaphorase by 1,2-dithiole-3-thione and increase of antitumour activity of bioreductive agents. Br J Cancer Suppl. 1996 Jul;27:S9–14. [PMC free article] [PubMed] [Google Scholar]
- Begleiter A., Leith M. K. Induction of DT-diaphorase by doxorubicin and combination therapy with mitomycin C in vitro. Biochem Pharmacol. 1995 Oct 12;50(8):1281–1286. doi: 10.1016/0006-2952(95)02014-4. [DOI] [PubMed] [Google Scholar]
- Begleiter A., Robotham E., Lacey G., Leith M. K. Increased sensitivity of quinone resistant cells to mitomycin C. Cancer Lett. 1989 Jun;45(3):173–176. doi: 10.1016/0304-3835(89)90073-6. [DOI] [PubMed] [Google Scholar]
- Begleiter A., Robotham E., Leith M. K. Role of NAD(P)H:(quinone acceptor) oxidoreductase (DT-diaphorase) in activation of mitomycin C under hypoxia. Mol Pharmacol. 1992 Apr;41(4):677–682. [PubMed] [Google Scholar]
- Belcourt M. F., Hodnick W. F., Rockwell S., Sartorelli A. C. Differential toxicity of mitomycin C and porfiromycin to aerobic and hypoxic Chinese hamster ovary cells overexpressing human NADPH:cytochrome c (P-450) reductase. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):456–460. doi: 10.1073/pnas.93.1.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky M., Jaiswal A. K. NAD(P)H:quinone oxidoreductase1 (DT-diaphorase) expression in normal and tumor tissues. Cancer Metastasis Rev. 1993 Jun;12(2):103–117. doi: 10.1007/BF00689804. [DOI] [PubMed] [Google Scholar]
- Benson A. M., Hunkeler M. J., Talalay P. Increase of NAD(P)H:quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5216–5220. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer R. E., Segura-Aguilar J. E., Ernster L. The anticancer enzyme DT diaphorase is induced selectively in liver during ascites hepatoma growth. Anticancer Res. 1988 Mar-Apr;8(2):233–238. [PubMed] [Google Scholar]
- Bueding E., Dolan P., Leroy J. P. The antischistosomal activity of oltipraz. Res Commun Chem Pathol Pharmacol. 1982 Aug;37(2):293–303. [PubMed] [Google Scholar]
- Egner P. A., Kensler T. W., Prestera T., Talalay P., Libby A. H., Joyner H. H., Curphey T. J. Regulation of phase 2 enzyme induction by oltipraz and other dithiolethiones. Carcinogenesis. 1994 Feb;15(2):177–181. doi: 10.1093/carcin/15.2.177. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons S. A., Workman P., Grever M., Paull K., Camalier R., Lewis A. D. Reductase enzyme expression across the National Cancer Institute Tumor cell line panel: correlation with sensitivity to mitomycin C and EO9. J Natl Cancer Inst. 1996 Mar 6;88(5):259–269. doi: 10.1093/jnci/88.5.259. [DOI] [PubMed] [Google Scholar]
- Gasdaska P. Y., Fisher H., Powis G. An alternatively spliced form of NQO1 (DT-diaphorase) messenger RNA lacking the putative quinone substrate binding site is present in human normal and tumor tissues. Cancer Res. 1995 Jun 15;55(12):2542–2547. [PubMed] [Google Scholar]
- Gustafson D. L., Pritsos C. A. Bioactivation of mitomycin C by xanthine dehydrogenase from EMT6 mouse mammary carcinoma tumors. J Natl Cancer Inst. 1992 Aug 5;84(15):1180–1185. doi: 10.1093/jnci/84.15.1180. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hodnick W. F., Sartorelli A. C. Reductive activation of mitomycin C by NADH:cytochrome b5 reductase. Cancer Res. 1993 Oct 15;53(20):4907–4912. [PubMed] [Google Scholar]
- Hortobagyi G. N. Mitomycin: its evolving role in the treatment of breast cancer. Oncology. 1993 Apr;50 (Suppl 1):1–8. [PubMed] [Google Scholar]
- Hu L. T., Stamberg J., Pan S. The NAD(P)H:quinone oxidoreductase locus in human colon carcinoma HCT 116 cells resistant to mitomycin C. Cancer Res. 1996 Nov 15;56(22):5253–5259. [PubMed] [Google Scholar]
- Jaiswal A. K., Burnett P., Adesnik M., McBride O. W. Nucleotide and deduced amino acid sequence of a human cDNA (NQO2) corresponding to a second member of the NAD(P)H:quinone oxidoreductase gene family. Extensive polymorphism at the NQO2 gene locus on chromosome 6. Biochemistry. 1990 Feb 20;29(7):1899–1906. doi: 10.1021/bi00459a034. [DOI] [PubMed] [Google Scholar]
- Jaiswal A. K. Human NAD(P)H:quinone oxidoreductase (NQO1) gene structure and induction by dioxin. Biochemistry. 1991 Nov 5;30(44):10647–10653. doi: 10.1021/bi00108a007. [DOI] [PubMed] [Google Scholar]
- Johnston J. B., Verburg L., Shore T., Williams M., Israels L. G., Begleiter A. Combination therapy with nucleoside analogs and alkylating agents. Leukemia. 1994 Apr;8 (Suppl 1):S140–S143. [PubMed] [Google Scholar]
- Kelloff G. J., Malone W. F., Boone C. W., Sigman C. C., Fay J. R. Progress in applied chemoprevention research. Semin Oncol. 1990 Aug;17(4):438–455. [PubMed] [Google Scholar]
- Kensler T. W., Helzlsouer K. J. Oltipraz: clinical opportunities for cancer chemoprevention. p. J Cell Biochem Suppl. 1995;22:101–107. doi: 10.1002/jcb.240590813. [DOI] [PubMed] [Google Scholar]
- Kensler T., Styczynski P., Groopman J., Helzlsouer K., Curphey T., Maxuitenko Y., Roebuck B. D. Mechanisms of chemoprotection by oltipraz. J Cell Biochem Suppl. 1992;16I:167–172. doi: 10.1002/jcb.240501331. [DOI] [PubMed] [Google Scholar]
- Klein D. S., Wilds P. R. Pulmonary toxicity of antineoplastic agents: anaesthetic and postoperative implications. Can Anaesth Soc J. 1983 Jul;30(4):399–405. doi: 10.1007/BF03007863. [DOI] [PubMed] [Google Scholar]
- Kuehl B. L., Paterson J. W., Peacock J. W., Paterson M. C., Rauth A. M. Presence of a heterozygous substitution and its relationship to DT-diaphorase activity. Br J Cancer. 1995 Sep;72(3):555–561. doi: 10.1038/bjc.1995.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Lafuente A., Trush M. A. Characterization of quinone reductase, glutathione and glutathione S-transferase in human myeloid cell lines: induction by 1,2-dithiole-3-thione and effects on hydroquinone-induced cytotoxicity. Life Sci. 1994;54(13):901–916. doi: 10.1016/0024-3205(94)00626-1. [DOI] [PubMed] [Google Scholar]
- Malkinson A. M., Siegel D., Forrest G. L., Gazdar A. F., Oie H. K., Chan D. C., Bunn P. A., Mabry M., Dykes D. J., Harrison S. D. Elevated DT-diaphorase activity and messenger RNA content in human non-small cell lung carcinoma: relationship to the response of lung tumor xenografts to mitomycin Cł. Cancer Res. 1992 Sep 1;52(17):4752–4757. [PubMed] [Google Scholar]
- Maxuitenko Y. Y., Curphey T. J., Kensler T. W., Roebuck B. D. Protection against aflatoxin B1-induced hepatic toxicity as short-term screen of cancer chemopreventive dithiolethiones. Fundam Appl Toxicol. 1996 Aug;32(2):250–259. doi: 10.1006/faat.1996.0128. [DOI] [PubMed] [Google Scholar]
- Mikami K., Naito M., Tomida A., Yamada M., Sirakusa T., Tsuruo T. DT-diaphorase as a critical determinant of sensitivity to mitomycin C in human colon and gastric carcinoma cell lines. Cancer Res. 1996 Jun 15;56(12):2823–2826. [PubMed] [Google Scholar]
- Nishiyama M., Saeki S., Aogi K., Hirabayashi N., Toge T. Relevance of DT-diaphorase activity to mitomycin C (MMC) efficacy on human cancer cells: differences in in vitro and in vivo systems. Int J Cancer. 1993 Apr 1;53(6):1013–1016. doi: 10.1002/ijc.2910530626. [DOI] [PubMed] [Google Scholar]
- O'Dwyer P. J., Szarka C. E., Yao K. S., Halbherr T. C., Pfeiffer G. R., Green F., Gallo J. M., Brennan J., Frucht H., Goosenberg E. B. Modulation of gene expression in subjects at risk for colorectal cancer by the chemopreventive dithiolethione oltipraz. J Clin Invest. 1996 Sep 1;98(5):1210–1217. doi: 10.1172/JCI118904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S. S., Andrews P. A., Glover C. J., Bachur N. R. Reductive activation of mitomycin C and mitomycin C metabolites catalyzed by NADPH-cytochrome P-450 reductase and xanthine oxidase. J Biol Chem. 1984 Jan 25;259(2):959–966. [PubMed] [Google Scholar]
- Patterson A. V., Robertson N., Houlbrook S., Stephens M. A., Adams G. E., Harris A. L., Stratford I. J., Carmichael J. The role of DT-diaphorase in determining the sensitivity of human tumor cells to tirapazamine (SR 4233). Int J Radiat Oncol Biol Phys. 1994 May 15;29(2):369–372. doi: 10.1016/0360-3016(94)90291-7. [DOI] [PubMed] [Google Scholar]
- Plumb J. A., Gerritsen M., Milroy R., Thomson P., Workman P. Relative importance of DT-diaphorase and hypoxia in the bioactivation of EO9 by human lung tumor cell lines. Int J Radiat Oncol Biol Phys. 1994 May 15;29(2):295–299. doi: 10.1016/0360-3016(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Powis G., Gasdaska P. Y., Gallegos A., Sherrill K., Goodman D. Over-expression of DT-diaphorase in transfected NIH 3T3 cells does not lead to increased anticancer quinone drug sensitivity: a questionable role for the enzyme as a target for bioreductively activated anticancer drugs. Anticancer Res. 1995 Jul-Aug;15(4):1141–1145. [PubMed] [Google Scholar]
- Prestera T., Zhang Y., Spencer S. R., Wilczak C. A., Talalay P. The electrophile counterattack response: protection against neoplasia and toxicity. Adv Enzyme Regul. 1993;33:281–296. doi: 10.1016/0065-2571(93)90024-8. [DOI] [PubMed] [Google Scholar]
- Prochaska H. J., Santamaria A. B. Direct measurement of NAD(P)H:quinone reductase from cells cultured in microtiter wells: a screening assay for anticarcinogenic enzyme inducers. Anal Biochem. 1988 Mar;169(2):328–336. doi: 10.1016/0003-2697(88)90292-8. [DOI] [PubMed] [Google Scholar]
- Riley R. J., Workman P. DT-diaphorase and cancer chemotherapy. Biochem Pharmacol. 1992 Apr 15;43(8):1657–1669. doi: 10.1016/0006-2952(92)90694-e. [DOI] [PubMed] [Google Scholar]
- Ross D., Siegel D., Beall H., Prakash A. S., Mulcahy R. T., Gibson N. W. DT-diaphorase in activation and detoxification of quinones. Bioreductive activation of mitomycin C. Cancer Metastasis Rev. 1993 Jun;12(2):83–101. doi: 10.1007/BF00689803. [DOI] [PubMed] [Google Scholar]
- Schellens J. H., Planting A. S., van Acker B. A., Loos W. J., de Boer-Dennert M., van der Burg M. E., Koier I., Krediet R. T., Stoter G., Verweij J. Phase I and pharmacologic study of the novel indoloquinone bioreductive alkylating cytotoxic drug E09. J Natl Cancer Inst. 1994 Jun 15;86(12):906–912. doi: 10.1093/jnci/86.12.906. [DOI] [PubMed] [Google Scholar]
- Schlager J. J., Powis G. Cytosolic NAD(P)H:(quinone-acceptor)oxidoreductase in human normal and tumor tissue: effects of cigarette smoking and alcohol. Int J Cancer. 1990 Mar 15;45(3):403–409. doi: 10.1002/ijc.2910450304. [DOI] [PubMed] [Google Scholar]
- Shao Y., Pardini L., Pardini R. S. Dietary menhaden oil enhances mitomycin C antitumor activity toward human mammary carcinoma MX-1. Lipids. 1995 Nov;30(11):1035–1045. doi: 10.1007/BF02536289. [DOI] [PubMed] [Google Scholar]
- Siegel D., Gibson N. W., Preusch P. C., Ross D. Metabolism of diaziquone by NAD(P)H:(quinone acceptor) oxidoreductase (DT-diaphorase): role in diaziquone-induced DNA damage and cytotoxicity in human colon carcinoma cells. Cancer Res. 1990 Nov 15;50(22):7293–7300. [PubMed] [Google Scholar]
- Smitskamp-Wilms E., Giaccone G., Pinedo H. M., van der Laan B. F., Peters G. J. DT-diaphorase activity in normal and neoplastic human tissues; an indicator for sensitivity to bioreductive agents? Br J Cancer. 1995 Oct;72(4):917–921. doi: 10.1038/bjc.1995.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel H. W., Dignam J. D. Purification and properties of NADPH-cytochrome P-450 reductase. Methods Enzymol. 1978;52:89–96. doi: 10.1016/s0076-6879(78)52009-0. [DOI] [PubMed] [Google Scholar]
- Talalay P. Mechanisms of induction of enzymes that protect against chemical carcinogenesis. Adv Enzyme Regul. 1989;28:237–250. doi: 10.1016/0065-2571(89)90074-5. [DOI] [PubMed] [Google Scholar]
- Traver R. D., Horikoshi T., Danenberg K. D., Stadlbauer T. H., Danenberg P. V., Ross D., Gibson N. W. NAD(P)H:quinone oxidoreductase gene expression in human colon carcinoma cells: characterization of a mutation which modulates DT-diaphorase activity and mitomycin sensitivity. Cancer Res. 1992 Feb 15;52(4):797–802. [PubMed] [Google Scholar]
- Traver R. D., Siegel D., Beall H. D., Phillips R. M., Gibson N. W., Franklin W. A., Ross D. Characterization of a polymorphism in NAD(P)H: quinone oxidoreductase (DT-diaphorase). Br J Cancer. 1997;75(1):69–75. doi: 10.1038/bjc.1997.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Williamson G. Transcriptional regulation of the human NAD(P)H:quinone oxidoreductase (NQO1) gene by monofunctional inducers. Biochim Biophys Acta. 1996 Jun 3;1307(1):104–110. doi: 10.1016/0167-4781(96)00028-0. [DOI] [PubMed] [Google Scholar]
- Waxman D. J. Glutathione S-transferases: role in alkylating agent resistance and possible target for modulation chemotherapy--a review. Cancer Res. 1990 Oct 15;50(20):6449–6454. [PubMed] [Google Scholar]
- Workman P., Stratford I. J. The experimental development of bioreductive drugs and their role in cancer therapy. Cancer Metastasis Rev. 1993 Jun;12(2):73–82. doi: 10.1007/BF00689802. [DOI] [PubMed] [Google Scholar]
- Xu B. H., Gupta V., Singh S. V. Characterization of a human bladder cancer cell line selected for resistance to mitomycin C. Int J Cancer. 1994 Sep 1;58(5):686–692. doi: 10.1002/ijc.2910580512. [DOI] [PubMed] [Google Scholar]
- Yao K. S., Godwin A. K., Johnson C., O'Dwyer P. J. Alternative splicing and differential expression of DT-diaphorase transcripts in human colon tumors and in peripheral mononuclear cells in response to mitomycin C treatment. Cancer Res. 1996 Apr 15;56(8):1731–1736. [PubMed] [Google Scholar]
- Yao K. S., O'Dwyer P. J. Involvement of NF-kappa B in the induction of NAD(P)H:quinone oxidoreductase (DT-diaphorase) by hypoxia, oltipraz and mitomycin C. Biochem Pharmacol. 1995 Jan 31;49(3):275–282. doi: 10.1016/0006-2952(94)00544-v. [DOI] [PubMed] [Google Scholar]


