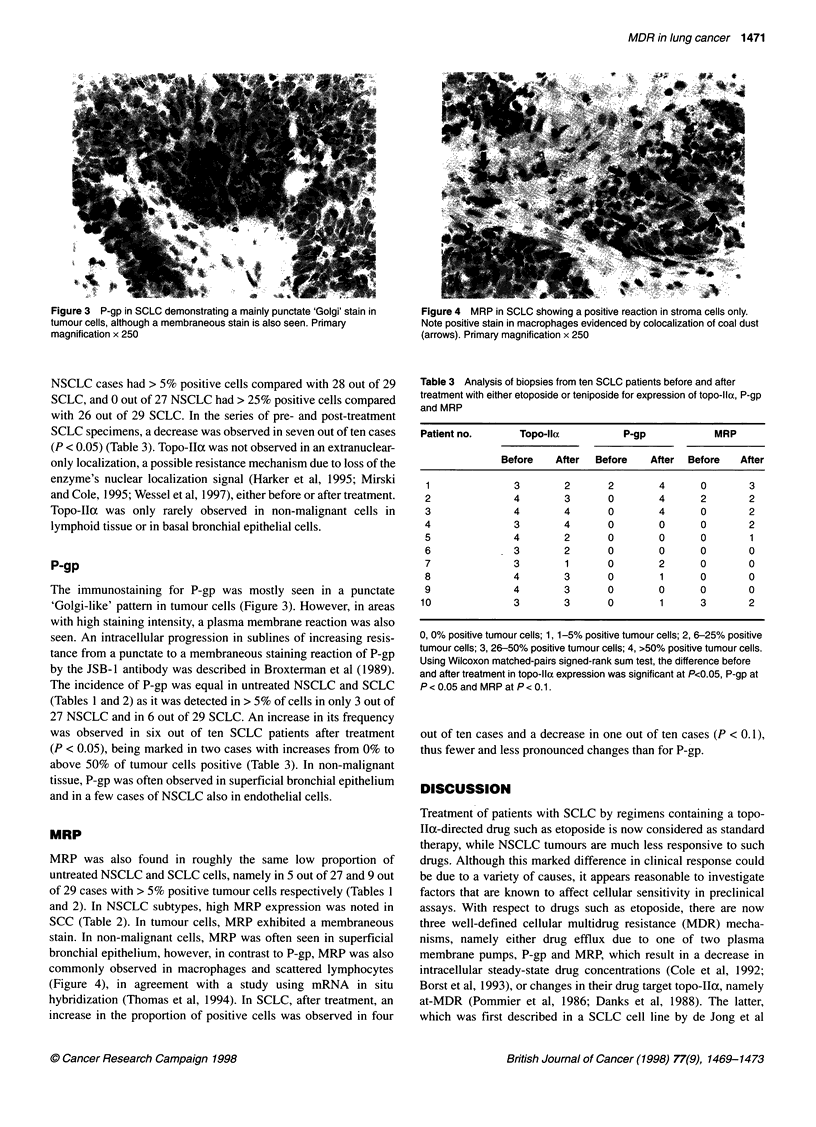
Abstract
Non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC) differ significantly in their clinical response to topoisomerase IIalpha (topo-IIalpha)-directed drugs, such as etoposide and teniposide, as NSCLC is virtually insensitive to single-agent therapy, while SCLC responds in two-thirds of cases. Preclinical studies have indicated that resistance to topo-IIalpha drugs depends on topo-IIalpha content and/or activity, the altered-topo-II multidrug resistance phenotype (at-MDR) and/or one of two different drug efflux pumps, P-glycoprotein (P-gp) and the multidrug resistance protein (MRP). Immunohistochemical analysis on paraffin-embedded tissue from 27 cases of untreated NSCLC and 29 cases of untreated SCLC (of which additional tumour biopsies after treatment with topo-IIalpha-directed drugs were available in ten cases) yielded the following results: NSCLC had significantly less topo-IIalpha than SCLC (P < 0.0001), as only 5 out of 27 NSCLC cases had > 5% positive cells compared with 28 out of 29 SCLC, and 0 out of 27 NSCLC had > 25% positive cells compared with 26 out of 29 SCLC. P-gp was detected in > 5% of cells in only 3 out of 27 NSCLC and in 6 out of 29 SCLC, and MRP in 5 out of 27 of NSCLC and 9 out of 29 SCLC. After treatment of patients with SCLC with either etoposide or teniposide, which are topo-IIalpha-directed drugs, there was an increase in MRP (P < 0.1) and P-gp (P < 0.05) positivity, while topo-IIalpha decreased (P < 0.05). In conclusion, the major difference between untreated NSCLC and SCLC was in topo-IIalpha content. In the small series of ten patients treated for SCLC, all three MDR phenotypes appeared to increase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Nakamura M., Ota E., Ozeki Y., Tamai S., Inoue H., Ueyama Y., Ogata T., Tamaoki N. Expression of the multidrug resistance gene (MDR1) in non-small cell lung cancer. Jpn J Cancer Res. 1994 May;85(5):536–541. doi: 10.1111/j.1349-7006.1994.tb02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck W. T., Grogan T. M., Willman C. L., Cordon-Cardo C., Parham D. M., Kuttesch J. F., Andreeff M., Bates S. E., Berard C. W., Boyett J. M. Methods to detect P-glycoprotein-associated multidrug resistance in patients' tumors: consensus recommendations. Cancer Res. 1996 Jul 1;56(13):3010–3020. [PubMed] [Google Scholar]
- Beer T. W., Rowlands D. C., Crocker J. Detection of the multidrug resistance marker P-glycoprotein by immunohistochemistry in malignant lung tumours. Thorax. 1996 May;51(5):526–529. doi: 10.1136/thx.51.5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boege F., Andersen A., Jensen S., Zeidler R., Kreipe H. Proliferation-associated nuclear antigen Ki-S1 is identical with topoisomerase II alpha. Delineation of a carboxy-terminal epitope with peptide antibodies. Am J Pathol. 1995 Jun;146(6):1302–1308. [PMC free article] [PubMed] [Google Scholar]
- Borst P., Schinkel A. H., Smit J. J., Wagenaar E., Van Deemter L., Smith A. J., Eijdems E. W., Baas F., Zaman G. J. Classical and novel forms of multidrug resistance and the physiological functions of P-glycoproteins in mammals. Pharmacol Ther. 1993 Nov;60(2):289–299. doi: 10.1016/0163-7258(93)90011-2. [DOI] [PubMed] [Google Scholar]
- Brock I., Hipfner D. R., Nielsen B. S., Jensen P. B., Deeley R. G., Cole S. P., Sehested M. Sequential coexpression of the multidrug resistance genes MRP and mdr1 and their products in VP-16 (etoposide)-selected H69 small cell lung cancer cells. Cancer Res. 1995 Feb 1;55(3):459–462. [PubMed] [Google Scholar]
- Broxterman H. J., Lankelma J., Pinedo H. M. How to probe clinical tumour samples for P-glycoprotein and multidrug resistance-associated protein. Eur J Cancer. 1996 Jun;32A(6):1024–1033. doi: 10.1016/0959-8049(96)00045-7. [DOI] [PubMed] [Google Scholar]
- Broxterman H. J., Pinedo H. M., Kuiper C. M., van der Hoeven J. J., de Lange P., Quak J. J., Scheper R. J., Keizer H. G., Schuurhuis G. J., Lankelma J. Immunohistochemical detection of P-glycoprotein in human tumor cells with a low degree of drug resistance. Int J Cancer. 1989 Feb 15;43(2):340–343. doi: 10.1002/ijc.2910430229. [DOI] [PubMed] [Google Scholar]
- Chen A. Y., Liu L. F. DNA topoisomerases: essential enzymes and lethal targets. Annu Rev Pharmacol Toxicol. 1994;34:191–218. doi: 10.1146/annurev.pa.34.040194.001203. [DOI] [PubMed] [Google Scholar]
- Chuman Y., Sumizawa T., Takebayashi Y., Niwa K., Yamada K., Haraguchi M., Furukawa T., Akiyama S., Aikou T. Expression of the multidrug-resistance-associated protein (MRP) gene in human colorectal, gastric and non-small-cell lung carcinomas. Int J Cancer. 1996 Apr 10;66(2):274–279. doi: 10.1002/(SICI)1097-0215(19960410)66:2<274::AID-IJC23>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992 Dec 4;258(5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Danks M. K., Schmidt C. A., Cirtain M. C., Suttle D. P., Beck W. T. Altered catalytic activity of and DNA cleavage by DNA topoisomerase II from human leukemic cells selected for resistance to VM-26. Biochemistry. 1988 Nov 29;27(24):8861–8869. doi: 10.1021/bi00424a026. [DOI] [PubMed] [Google Scholar]
- Flens M. J., Izquierdo M. A., Scheffer G. L., Fritz J. M., Meijer C. J., Scheper R. J., Zaman G. J. Immunochemical detection of the multidrug resistance-associated protein MRP in human multidrug-resistant tumor cells by monoclonal antibodies. Cancer Res. 1994 Sep 1;54(17):4557–4563. [PubMed] [Google Scholar]
- Giaccone G., van Ark-Otte J., Rubio G. J., Gazdar A. F., Broxterman H. J., Dingemans A. M., Flens M. J., Scheper R. J., Pinedo H. M. MRP is frequently expressed in human lung-cancer cell lines, in non-small-cell lung cancer and in normal lungs. Int J Cancer. 1996 Jun 11;66(6):760–767. doi: 10.1002/(SICI)1097-0215(19960611)66:6<760::AID-IJC9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Guinee D. G., Jr, Holden J. A., Benfield J. R., Woodward M. L., Przygodzki R. M., Fishback N. F., Koss M. N., Travis W. D. Comparison of DNA topoisomerase II alpha expression in small cell and nonsmall cell carcinoma of the lung. In search of a mechanism of chemotherapeutic response. Cancer. 1996 Aug 15;78(4):729–735. doi: 10.1002/(SICI)1097-0142(19960815)78:4<729::AID-CNCR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Hansen H. H. Management of small-cell cancer of the lung. Lancet. 1992 Apr 4;339(8797):846–849. doi: 10.1016/0140-6736(92)90287-d. [DOI] [PubMed] [Google Scholar]
- Harker W. G., Slade D. L., Parr R. L., Feldhoff P. W., Sullivan D. M., Holguin M. H. Alterations in the topoisomerase II alpha gene, messenger RNA, and subcellular protein distribution as well as reduced expression of the DNA topoisomerase II beta enzyme in a mitoxantrone-resistant HL-60 human leukemia cell line. Cancer Res. 1995 Apr 15;55(8):1707–1716. [PubMed] [Google Scholar]
- Hellemans P., van Dam P. A., Geyskens M., van Oosterom A. T., Buytaert P., Van Marck E. Immunohistochemical study of topoisomerase II-alpha expression in primary ductal carcinoma of the breast. J Clin Pathol. 1995 Feb;48(2):147–150. doi: 10.1136/jcp.48.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmayer T. A., Hilsenbeck S., Von Hoff D. D., Roninson I. B. Clinical correlates of MDR1 (P-glycoprotein) gene expression in ovarian and small-cell lung carcinomas. J Natl Cancer Inst. 1992 Oct 7;84(19):1486–1491. doi: 10.1093/jnci/84.19.1486. [DOI] [PubMed] [Google Scholar]
- Järvinen T. A., Kononen J., Pelto-Huikko M., Isola J. Expression of topoisomerase IIalpha is associated with rapid cell proliferation, aneuploidy, and c-erbB2 overexpression in breast cancer. Am J Pathol. 1996 Jun;148(6):2073–2082. [PMC free article] [PubMed] [Google Scholar]
- Kreipe H., Alm P., Olsson H., Hauberg M., Fischer L., Parwaresch R. Prognostic significance of a formalin-resistant nuclear proliferation antigen in mammary carcinomas as determined by the monoclonal antibody Ki-S1. Am J Pathol. 1993 Feb;142(2):651–657. [PMC free article] [PubMed] [Google Scholar]
- Mirski S. E., Cole S. P. Cytoplasmic localization of a mutant M(r) 160,000 topoisomerase II alpha is associated with the loss of putative bipartite nuclear localization signals in a drug-resistant human lung cancer cell line. Cancer Res. 1995 May 15;55(10):2129–2134. [PubMed] [Google Scholar]
- Narasaki F., Matsuo I., Ikuno N., Fukuda M., Soda H., Oka M. Multidrug resistance-associated protein (MRP) gene expression in human lung cancer. Anticancer Res. 1996 Jul-Aug;16(4A):2079–2082. [PubMed] [Google Scholar]
- Nitiss J. L., Liu Y. X., Hsiung Y. A temperature sensitive topoisomerase II allele confers temperature dependent drug resistance on amsacrine and etoposide: a genetic system for determining the targets of topoisomerase II inhibitors. Cancer Res. 1993 Jan 1;53(1):89–93. [PubMed] [Google Scholar]
- Nooter K., Bosman F. T., Burger H., van Wingerden K. E., Flens M. J., Scheper R. J., Oostrum R. G., Boersma A. W., van der Gaast A., Stoter G. Expression of the multidrug resistance-associated protein (MRP) gene in primary non-small-cell lung cancer. Ann Oncol. 1996 Jan;7(1):75–81. doi: 10.1093/oxfordjournals.annonc.a010484. [DOI] [PubMed] [Google Scholar]
- Oberli-Schrämmli A. E., Joncourt F., Stadler M., Altermatt H. J., Buser K., Ris H. B., Schmid U., Cerny T. Parallel assessment of glutathione-based detoxifying enzymes, O6-alkylguanine-DNA alkyltransferase and P-glycoprotein as indicators of drug resistance in tumor and normal lung of patients with lung cancer. Int J Cancer. 1994 Dec 1;59(5):629–636. doi: 10.1002/ijc.2910590509. [DOI] [PubMed] [Google Scholar]
- Ota E., Abe Y., Oshika Y., Ozeki Y., Iwasaki M., Inoue H., Yamazaki H., Ueyama Y., Takagi K., Ogata T. Expression of the multidrug resistance-associated protein (MRP) gene in non-small-cell lung cancer. Br J Cancer. 1995 Sep;72(3):550–554. doi: 10.1038/bjc.1995.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y., Kerrigan D., Schwartz R. E., Swack J. A., McCurdy A. Altered DNA topoisomerase II activity in Chinese hamster cells resistant to topoisomerase II inhibitors. Cancer Res. 1986 Jun;46(6):3075–3081. [PubMed] [Google Scholar]
- Segawa Y., Ohnoshi T., Hiraki S., Ueoka H., Kiura K., Kamei H., Tabata M., Shibayama T., Miyatake K., Genba K. Immunohistochemical detection of P-glycoprotein and carcinoembryonic antigen in small cell lung cancer: with reference to predictability of response to chemotherapy. Acta Med Okayama. 1993 Jun;47(3):181–189. doi: 10.18926/AMO/31590. [DOI] [PubMed] [Google Scholar]
- Sugawara I., Yamada H., Nakamura H., Sumizawa T., Akiyama S., Masunaga A., Itoyama S. Preferential expression of the multidrug-resistance-associated protein (MRP) in adenocarcinoma of the lung. Int J Cancer. 1995 Oct 20;64(5):322–325. doi: 10.1002/ijc.2910640507. [DOI] [PubMed] [Google Scholar]
- Tabata M., Ohnoshi T., Ueoka H., Kiura K., Kimura I. MDR1 gene expression and treatment outcome in small cell lung cancer: MDR1 gene expression as an independent prognostic factor. Acta Med Okayama. 1993 Aug;47(4):243–248. doi: 10.18926/AMO/31553. [DOI] [PubMed] [Google Scholar]
- Thomas G. A., Barrand M. A., Stewart S., Rabbitts P. H., Williams E. D., Twentyman P. R. Expression of the multidrug resistance-associated protein (MRP) gene in human lung tumours and normal tissue as determined by in situ hybridisation. Eur J Cancer. 1994;30A(11):1705–1709. doi: 10.1016/0959-8049(94)00290-l. [DOI] [PubMed] [Google Scholar]
- Tuccari G., Rizzo A., Giuffrè G., Barresi G. Immunocytochemical detection of DNA topoisomerase type II in primary breast carcinomas: correlation with clinico-pathological features. Virchows Arch A Pathol Anat Histopathol. 1993;423(1):51–55. doi: 10.1007/BF01606432. [DOI] [PubMed] [Google Scholar]
- Volm M., Mattern J., Samsel B. Overexpression of P-glycoprotein and glutathione S-transferase-pi in resistant non-small cell lung carcinomas of smokers. Br J Cancer. 1991 Oct;64(4):700–704. doi: 10.1038/bjc.1991.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C. D., Latham M. D., Lock R. B., Sullivan D. M. Attenuated topoisomerase II content directly correlates with a low level of drug resistance in a Chinese hamster ovary cell line. Cancer Res. 1991 Dec 15;51(24):6543–6549. [PubMed] [Google Scholar]
- Yamazaki K., Isobe H., Hanada T., Sukoh N., Ogura S., Kawakami Y. Quantitative immunocytochemical assays of topoisomerase II in lung adenocarcinoma cell lines. Correlation to topoisomerase II alpha content and topoisomerase II catalytic activity. Acta Oncol. 1996;35(4):417–423. doi: 10.3109/02841869609109915. [DOI] [PubMed] [Google Scholar]
- de Jong S., Zijlstra J. G., de Vries E. G., Mulder N. H. Reduced DNA topoisomerase II activity and drug-induced DNA cleavage activity in an adriamycin-resistant human small cell lung carcinoma cell line. Cancer Res. 1990 Jan 15;50(2):304–309. [PubMed] [Google Scholar]






