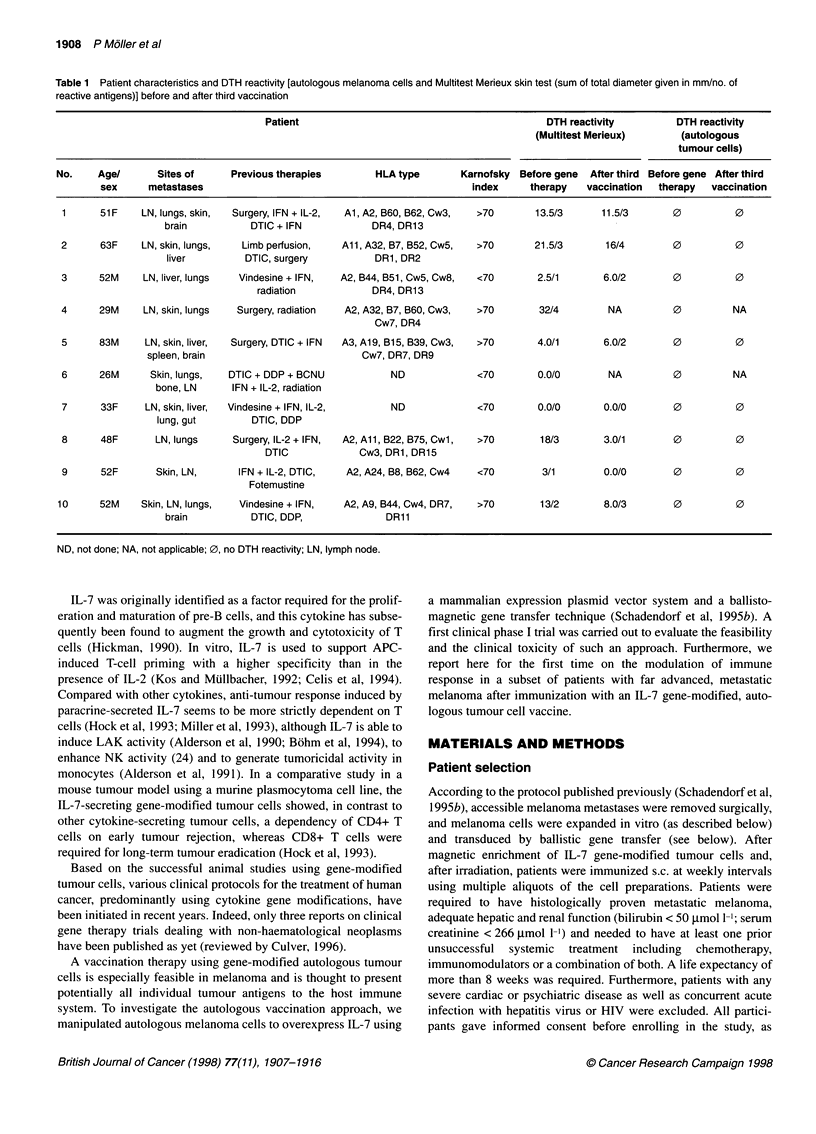
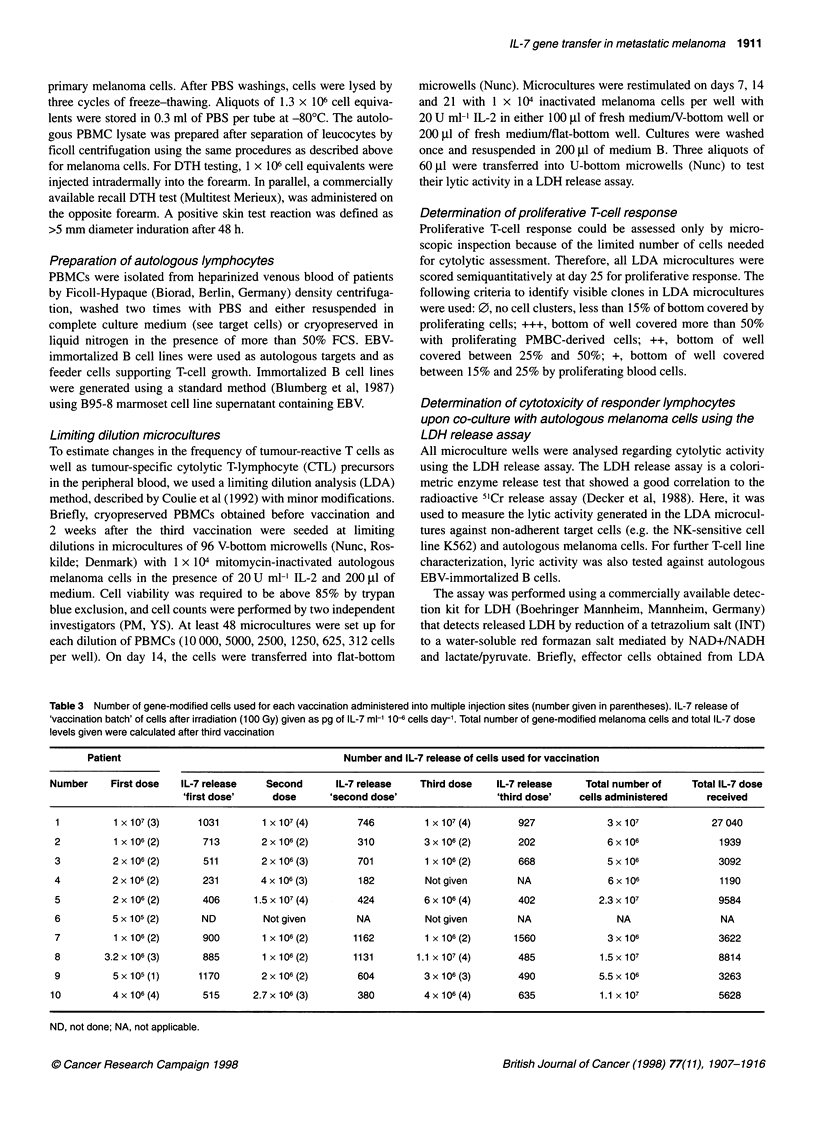
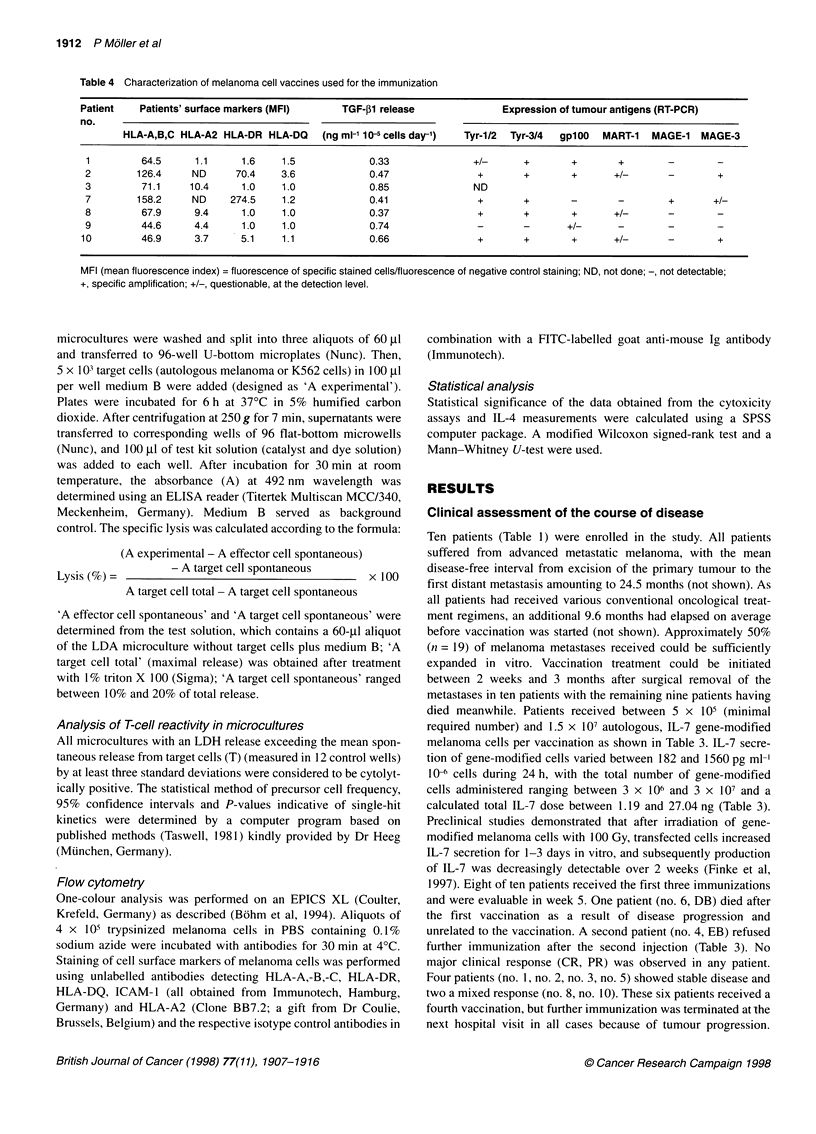
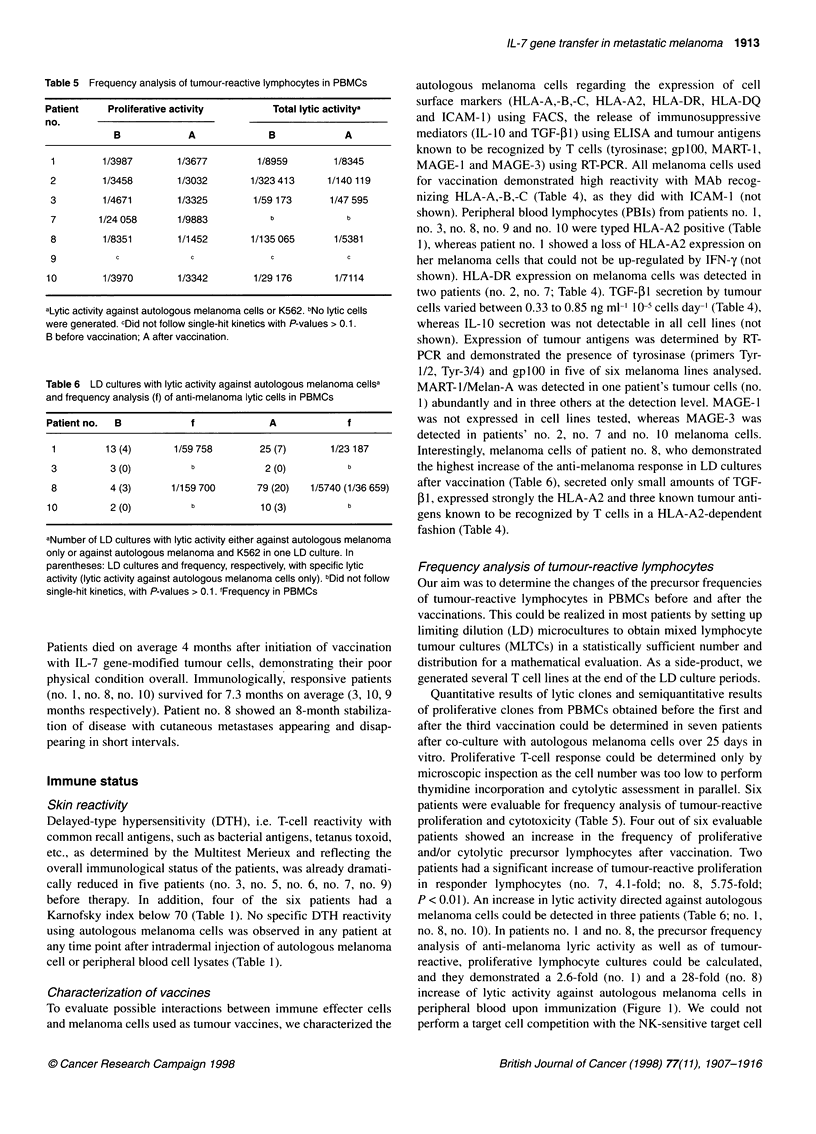
Abstract
Recently, cytokine gene transfer into tumour cells has been shown to mediate tumour regression in animal models via immunomodulation. Consequently, a number of clinical protocols have been developed to treat cancer patients with cytokine gene-modified tumour cells. Here, we report the results of a clinical phase I trial using for the first time autologous, interleukin 7 gene-modified tumour cells for vaccination of ten patients with disseminated malignant melanoma. Melanoma cells were expanded in vitro from surgically removed metastases, transduced by a ballistic gene transfer technique and were then injected after in vitro irradiation s.c. at weekly intervals. Clinically, there was no major toxicity except for mild fever, and no major clinical response towards vaccination was observed. Eight of ten patients completed the initial three s.c. vaccinations and were eligible for immunological evaluation. Post vaccination, peripheral mononuclear cells (PBMCs) were found to contain an increased number of tumour-reactive proliferative as well as cytolytic cells, as determined by a limiting dilution analysis. In three of six patients, the frequencies of anti-melanoma cytolytic precursor cells increased between 2.6- and 28-fold. Two of these patients showed a minor clinical response. Analysis of the autologous tumour cell vaccines regarding IL-7 secretion after gene transfer, HLA class I and class II cell surface expression, secretion of immunosuppressive mediators (TGF-beta1, IL-10) and various melanoma-associated tumour antigens revealed a very diverse expression profile. In conclusion, vaccination using gene-modified autologous melanoma cells induced immunological changes in a group of advanced, terminally ill patients. These changes can be interpreted as an increased anti-tumour immune response. However, immunological modulation was most pronounced in patients in good physical condition. Therefore, patients with minimal tumour load or minimal residual disease might preferentially benefit from tumour cell vaccination in further studies. In order to evaluate the effects of the cytokine gene-modified tumour cell vaccines more precisely, an antigenically better defined vaccine is needed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A pilot study of immunization with HLA-A2 matched allogeneic melanoma cells that secrete interleukin-2 in patients with metastatic melanoma. Hum Gene Ther. 1992 Dec;3(6):677–690. doi: 10.1089/hum.1992.3.6-677. [DOI] [PubMed] [Google Scholar]
- Ahmann D. L., Creagan E. T., Hahn R. G., Edmonson J. H., Bisel H. F., Schaid D. J. Complete responses and long-term survivals after systemic chemotherapy for patients with advanced malignant melanoma. Cancer. 1989 Jan 15;63(2):224–227. doi: 10.1002/1097-0142(19890115)63:2<224::aid-cncr2820630203>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Alderson M. R., Sassenfeld H. M., Widmer M. B. Interleukin 7 enhances cytolytic T lymphocyte generation and induces lymphokine-activated killer cells from human peripheral blood. J Exp Med. 1990 Aug 1;172(2):577–587. doi: 10.1084/jem.172.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson M. R., Tough T. W., Ziegler S. F., Grabstein K. H. Interleukin 7 induces cytokine secretion and tumoricidal activity by human peripheral blood monocytes. J Exp Med. 1991 Apr 1;173(4):923–930. doi: 10.1084/jem.173.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg R. S., Paradis T., Byington R., Henle W., Hirsch M. S., Schooley R. T. Effects of human immunodeficiency virus on the cellular immune response to Epstein-Barr virus in homosexual men: characterization of the cytotoxic response and lymphokine production. J Infect Dis. 1987 May;155(5):877–890. doi: 10.1093/infdis/155.5.877. [DOI] [PubMed] [Google Scholar]
- Böhm M., Möller P., Kalbfleisch U., Worm M., Czarnetzki B. M., Schadendorf D. Lysis of allogeneic and autologous melanoma cells by IL-7-induced lymphokine-activated killer cells. Br J Cancer. 1994 Jul;70(1):54–59. doi: 10.1038/bjc.1994.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis E., Tsai V., Crimi C., DeMars R., Wentworth P. A., Chesnut R. W., Grey H. M., Sette A., Serra H. M. Induction of anti-tumor cytotoxic T lymphocytes in normal humans using primary cultures and synthetic peptide epitopes. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2105–2109. doi: 10.1073/pnas.91.6.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M. P., Forni G. Cytokine gene transfer in tumor inhibition and tumor therapy: where are we now? Immunol Today. 1994 Feb;15(2):48–51. doi: 10.1016/0167-5699(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Coulie P. G., Somville M., Lehmann F., Hainaut P., Brasseur F., Devos R., Boon T. Precursor frequency analysis of human cytolytic T lymphocytes directed against autologous melanoma cells. Int J Cancer. 1992 Jan 21;50(2):289–297. doi: 10.1002/ijc.2910500220. [DOI] [PubMed] [Google Scholar]
- Culver K. W. Measuring success in clinical gene therapy research. Mol Med Today. 1996 Jun;2(6):234–236. doi: 10.1016/1357-4310(96)88803-4. [DOI] [PubMed] [Google Scholar]
- Decker T., Lohmann-Matthes M. L. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods. 1988 Nov 25;115(1):61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Pardoll D. M., Itaya T., Golumbek P., Levitsky H. I., Simons J. W., Karasuyama H., Vogelstein B., Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990 Feb 9;60(3):397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- Ferrone S. Melanoma, immune surveillance, and immunotherapy. J Clin Invest. 1994 Apr;93(4):1351–1352. doi: 10.1172/JCI117108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion M. C., Bradley A. J., Devine D. V., Décary F., Chartrand P. Autoreactive T cells in healthy individuals show tolerance in vitro with characteristics similar to but distinct from clonal anergy. Eur J Immunol. 1995 Nov;25(11):3123–3127. doi: 10.1002/eji.1830251120. [DOI] [PubMed] [Google Scholar]
- Herr W., Wölfel T., Heike M., Meyer zum Büschenfelde K. H., Knuth A. Frequency analysis of tumor-reactive cytotoxic T lymphocytes in peripheral blood of a melanoma patient vaccinated with autologous tumor cells. Cancer Immunol Immunother. 1994 Aug;39(2):93–99. doi: 10.1007/BF01525314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman C. J., Crim J. A., Mostowski H. S., Siegel J. P. Regulation of human cytotoxic T lymphocyte development by IL-7. J Immunol. 1990 Oct 15;145(8):2415–2420. [PubMed] [Google Scholar]
- Ho V. C., Sober A. J. Therapy for cutaneous melanoma: an update. J Am Acad Dermatol. 1990 Feb;22(2 Pt 1):159–176. doi: 10.1016/0190-9622(90)70019-e. [DOI] [PubMed] [Google Scholar]
- Hock H., Dorsch M., Kunzendorf U., Qin Z., Diamantstein T., Blankenstein T. Mechanisms of rejection induced by tumor cell-targeted gene transfer of interleukin 2, interleukin 4, interleukin 7, tumor necrosis factor, or interferon gamma. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2774–2778. doi: 10.1073/pnas.90.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton A. N. Cancer antigens: immune recognition of self and altered self. J Exp Med. 1994 Jul 1;180(1):1–4. doi: 10.1084/jem.180.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Delgado C. H., Robbins P. F., Sakaguchi K., Appella E., Yannelli J. R., Adema G. J., Miki T., Rosenberg S. A. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos F. J., Müllbacher A. Induction of primary anti-viral cytotoxic T cells by in vitro stimulation with short synthetic peptide and interleukin-7. Eur J Immunol. 1992 Dec;22(12):3183–3185. doi: 10.1002/eji.1830221224. [DOI] [PubMed] [Google Scholar]
- Mackensen A., Carcelain G., Viel S., Raynal M. C., Michalaki H., Triebel F., Bosq J., Hercend T. Direct evidence to support the immunosurveillance concept in a human regressive melanoma. J Clin Invest. 1994 Apr;93(4):1397–1402. doi: 10.1172/JCI117116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. R., McBride W. H., Dubinett S. M., Dougherty G. J., Thacker J. D., Shau H., Kohn D. B., Moen R. C., Walker M. J., Chiu R. Transduction of human melanoma cell lines with the human interleukin-7 gene using retroviral-mediated gene transfer: comparison of immunologic properties with interleukin-2. Blood. 1993 Dec 15;82(12):3686–3694. [PubMed] [Google Scholar]
- Namen A. E., Schmierer A. E., March C. J., Overell R. W., Park L. S., Urdal D. L., Mochizuki D. Y. B cell precursor growth-promoting activity. Purification and characterization of a growth factor active on lymphocyte precursors. J Exp Med. 1988 Mar 1;167(3):988–1002. doi: 10.1084/jem.167.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naume B., Espevik T. Effects of IL-7 and IL-2 on highly enriched CD56+ natural killer cells. A comparative study. J Immunol. 1991 Oct 1;147(7):2208–2214. [PubMed] [Google Scholar]
- Pardoll D. M. Paracrine cytokine adjuvants in cancer immunotherapy. Annu Rev Immunol. 1995;13:399–415. doi: 10.1146/annurev.iy.13.040195.002151. [DOI] [PubMed] [Google Scholar]
- Robbins P. F., el-Gamil M., Kawakami Y., Stevens E., Yannelli J. R., Rosenberg S. A. Recognition of tyrosinase by tumor-infiltrating lymphocytes from a patient responding to immunotherapy. Cancer Res. 1994 Jun 15;54(12):3124–3126. [PubMed] [Google Scholar]
- Schadendorf D., Czarnetzki B. M., Wittig B. Interleukin-7, interleukin-12, and GM-CSF gene transfer in patients with metastatic melanoma. J Mol Med (Berl) 1995 Sep;73(9):473–477. doi: 10.1007/BF00202266. [DOI] [PubMed] [Google Scholar]
- Schadendorf D., Fichtner I., Makki A., Alijagic S., Küpper M., Mrowietz U., Henz B. M. Metastatic potential of human melanoma cells in nude mice--characterisation of phenotype, cytokine secretion and tumour-associated antigens. Br J Cancer. 1996 Jul;74(2):194–199. doi: 10.1038/bjc.1996.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadendorf D., Makki A., Stahr C., van Dyck A., Wanner R., Scheffer G. L., Flens M. J., Scheper R., Henz B. M. Membrane transport proteins associated with drug resistance expressed in human melanoma. Am J Pathol. 1995 Dec;147(6):1545–1552. [PMC free article] [PubMed] [Google Scholar]
- Schadendorf D., Worm M., Algermissen B., Kohlmus C. M., Czarnetzki B. M. Chemosensitivity testing of human malignant melanoma. A retrospective analysis of clinical response and in vitro drug sensitivity. Cancer. 1994 Jan 1;73(1):103–108. doi: 10.1002/1097-0142(19940101)73:1<103::aid-cncr2820730119>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wolf G., Schmidt-Wolf I. G. Cytokines and clinical gene therapy. Eur J Immunol. 1995 Apr;25(4):1137–1140. doi: 10.1002/eji.1830250445. [DOI] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Tepper R. I., Pattengale P. K., Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989 May 5;57(3):503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- Vieweg J., Gilboa E. Considerations for the use of cytokine-secreting tumor cell preparations for cancer treatment. Cancer Invest. 1995;13(2):193–201. doi: 10.3109/07357909509011690. [DOI] [PubMed] [Google Scholar]


