Abstract
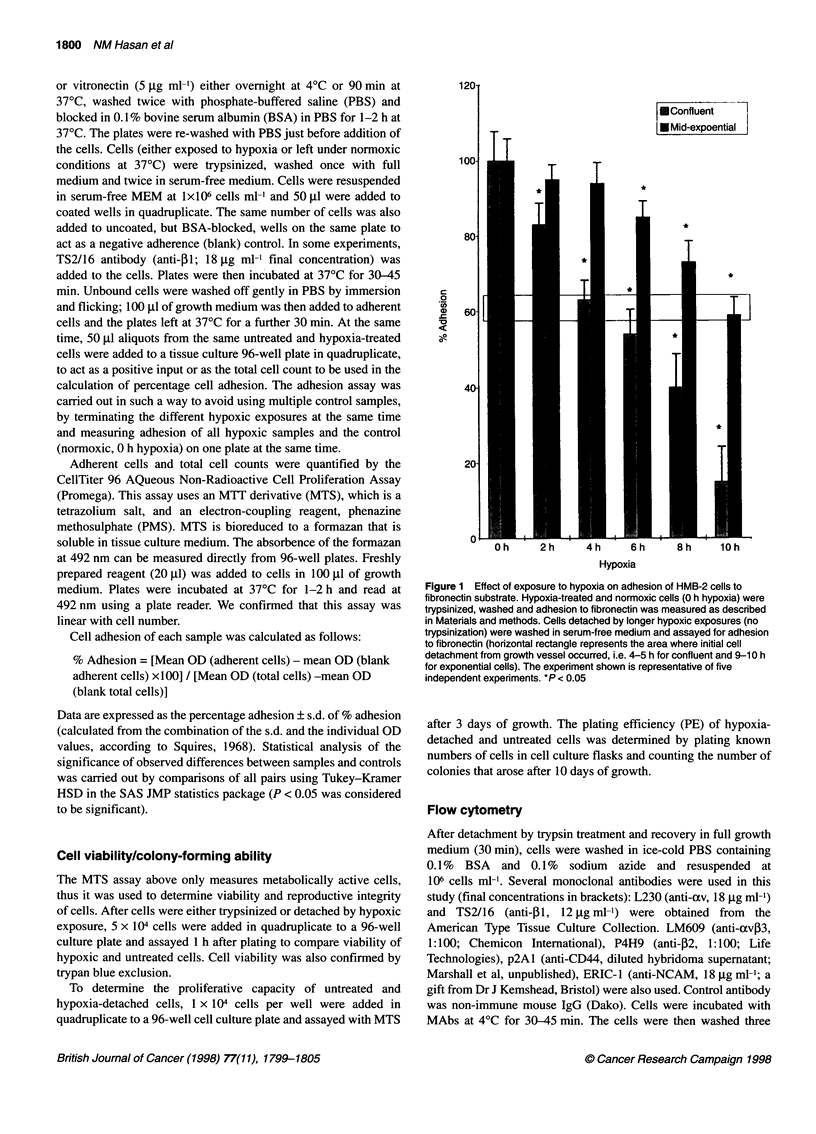
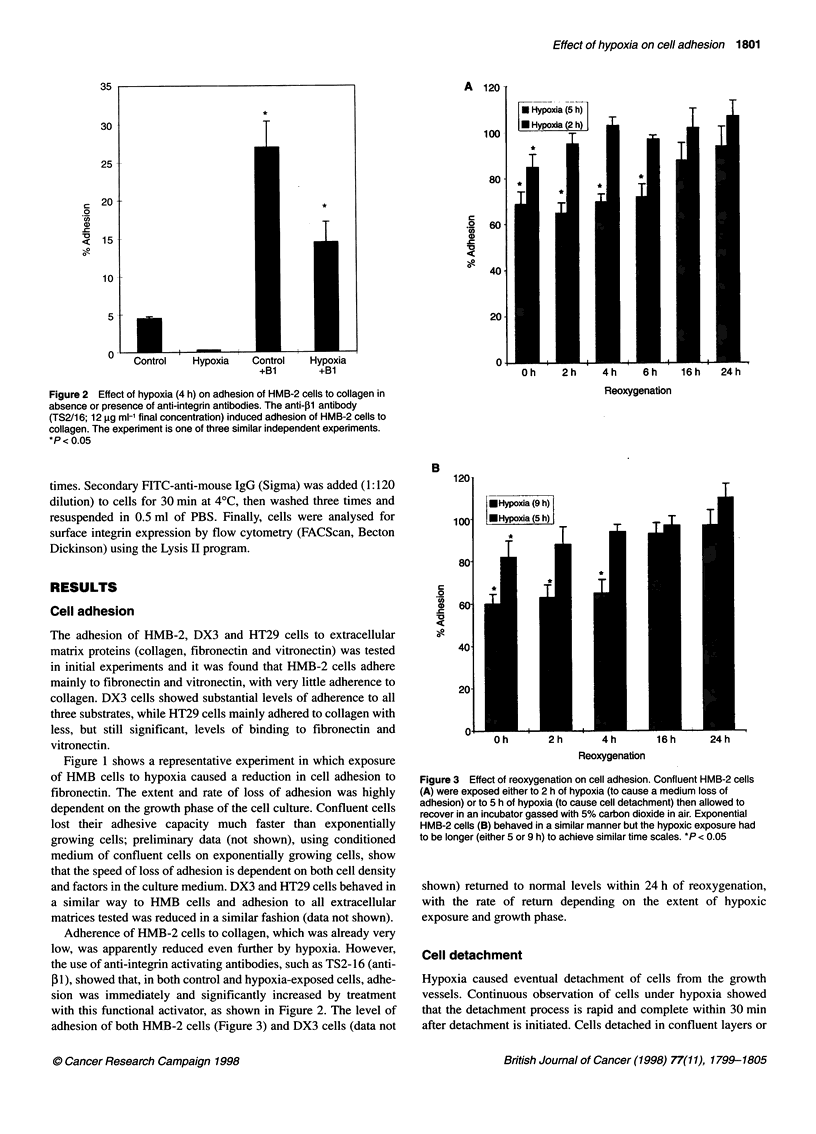
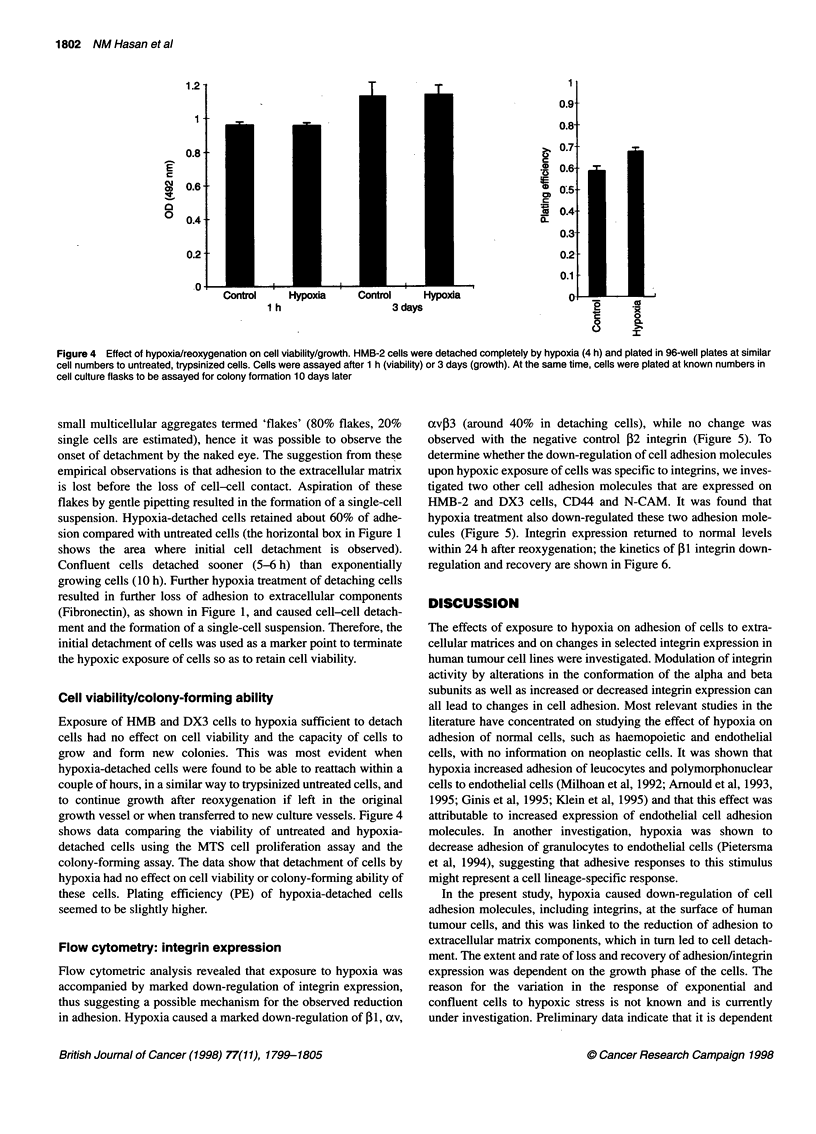
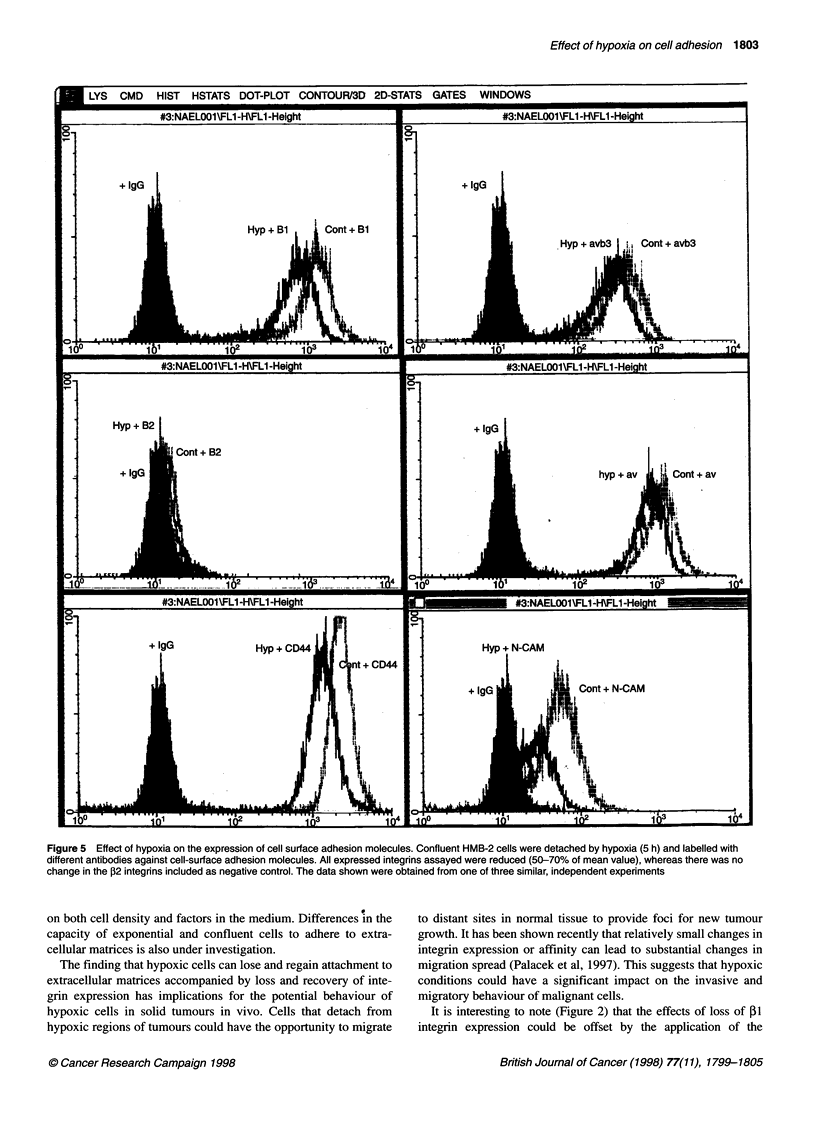
The effects of acute hypoxia on integrin expression and adhesion to extracellular matrix proteins were investigated in two human melanoma cell lines, HMB-2 and DX3, and a human adenocarcinoma cell line, HT29. Exposure to hypoxia caused a significant down-regulation of cell surface integrins and an associated decrease in cell adhesion. Loss of cell adhesion and integrin expression were transient and levels returned to normal within 24 h of reoxygenation. Other cell adhesion molecules, such as CD44 and N-CAM, were also down-regulated after exposure of cells to hypoxia. Acute exposure to hypoxia of cells at confluence caused rapid cell detachment. Cell detachment preceded loss of viability. Detached HMB-2 and DX3 cells completely recovered upon reoxygenation, and floating cells re-attached and continued to grow irrespective of whether they were left in the original glass dishes or transferred to new culture vessels, while detached HT29 cells partly recovered upon reoxygenation. Cell detachment after decreased adhesion appears to be a stress response, which may be a factor enabling malignant cells to escape hypoxia in vivo, with the potential to form new foci of tumour growth.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnould T., Michiels C., Janssens D., Delaive E., Remacle J. Hypoxia induces PMN adherence to umbilical vein endothelium. Cardiovasc Res. 1995 Dec;30(6):1009–1016. [PubMed] [Google Scholar]
- Brizel D. M., Scully S. P., Harrelson J. M., Layfield L. J., Bean J. M., Prosnitz L. R., Dewhirst M. W. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996 Mar 1;56(5):941–943. [PubMed] [Google Scholar]
- Ginis I., Mentzer S. J., Li X., Faller D. V. Characterization of a hypoxia-responsive adhesion molecule for leukocytes on human endothelial cells. J Immunol. 1995 Jul 15;155(2):802–810. [PubMed] [Google Scholar]
- Graeber T. G., Osmanian C., Jacks T., Housman D. E., Koch C. J., Lowe S. W., Giaccia A. J. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996 Jan 4;379(6560):88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- Hasan N. M., Parker P. J., Adams G. E. Induction and phosphorylation of protein kinase C-alpha and mitogen-activated protein kinase by hypoxia and by radiation in Chinese hamster V79 cells. Radiat Res. 1996 Feb;145(2):128–133. [PubMed] [Google Scholar]
- Hockel M., Schlenger K., Aral B., Mitze M., Schaffer U., Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996 Oct 1;56(19):4509–4515. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Juliano R. L. Membrane receptors for extracellular matrix macromolecules: relationship to cell adhesion and tumor metastasis. Biochim Biophys Acta. 1987 Nov 25;907(3):261–278. doi: 10.1016/0304-419x(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Varner J. A. Adhesion molecules in cancer: the role of integrins. Curr Opin Cell Biol. 1993 Oct;5(5):812–818. doi: 10.1016/0955-0674(93)90030-t. [DOI] [PubMed] [Google Scholar]
- Klein C. L., Köhler H., Bittinger F., Otto M., Hermanns I., Kirkpatrick C. J. Comparative studies on vascular endothelium in vitro. 2. Hypoxia: its influences on endothelial cell proliferation and expression of cell adhesion molecules. Pathobiology. 1995;63(1):1–8. doi: 10.1159/000163928. [DOI] [PubMed] [Google Scholar]
- Lawrence T. S., Davis M. A., Loney T. L. Fluoropyrimidine-mediated radiosensitization depends on cyclin E-dependent kinase activation. Cancer Res. 1996 Jul 15;56(14):3203–3206. [PubMed] [Google Scholar]
- Lomax M. E., Barnes D. M., Gilchrist R., Picksley S. M., Varley J. M., Camplejohn R. S. Two functional assays employed to detect an unusual mutation in the oligomerisation domain of p53 in a Li-Fraumeni like family. Oncogene. 1997 Apr 17;14(15):1869–1874. doi: 10.1038/sj.onc.1201133. [DOI] [PubMed] [Google Scholar]
- Milhoan K. A., Lane T. A., Bloor C. M. Hypoxia induces endothelial cells to increase their adherence for neutrophils: role of PAF. Am J Physiol. 1992 Sep;263(3 Pt 2):H956–H962. doi: 10.1152/ajpheart.1992.263.3.H956. [DOI] [PubMed] [Google Scholar]
- Montano X., Shamsher M., Whitehead P., Dawson K., Newton J. Analysis of p53 in human cutaneous melanoma cell lines. Oncogene. 1994 May;9(5):1455–1459. [PubMed] [Google Scholar]
- Onoda J. M., Piechocki M. P., Honn K. V. Radiation-induced increase in expression of the alpha IIb beta 3 integrin in melanoma cells: effects on metastatic potential. Radiat Res. 1992 Jun;130(3):281–288. [PubMed] [Google Scholar]
- Palecek S. P., Loftus J. C., Ginsberg M. H., Lauffenburger D. A., Horwitz A. F. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997 Feb 6;385(6616):537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Pietersma A., de Jong N., Koster J. F., Sluiter W. Extreme hypoxia decreases the adherence of granulocytes to endothelial cells in vitro. Ann N Y Acad Sci. 1994 Jun 17;723:486–487. [PubMed] [Google Scholar]
- Shrieve D. C., Deen D. F., Harris J. W. Effects of extreme hypoxia on the growth and viability of EMT6/SF mouse tumor cells in vitro. Cancer Res. 1983 Aug;43(8):3521–3527. [PubMed] [Google Scholar]
- Spiro I. J., Rice G. C., Durand R. E., Stickler R., Ling C. C. Cell killing, radiosensitization and cell cycle redistribution induced by chronic hypoxia. Int J Radiat Oncol Biol Phys. 1984 Aug;10(8):1275–1280. doi: 10.1016/0360-3016(84)90332-8. [DOI] [PubMed] [Google Scholar]
- Stratford I. J., Williamson C., Adams G. E. Combination studies with misonidazole and a cis-platinum complex: cytotoxicity and radiosensitization in vitro. Br J Cancer. 1980 Apr;41(4):517–522. doi: 10.1038/bjc.1980.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R. M., Keng P., Conroy P. J., McDermott D., Bareham B. J., Passalacqua W. In vitro hypoxic cytotoxicity of nitroimidazoles: uptake and cell cycle phase specificity. Int J Radiat Oncol Biol Phys. 1982 Mar-Apr;8(3-4):745–748. doi: 10.1016/0360-3016(82)90726-x. [DOI] [PubMed] [Google Scholar]
- Yao K. S., Clayton M., O'Dwyer P. J. Apoptosis in human adenocarcinoma HT29 cells induced by exposure to hypoxia. J Natl Cancer Inst. 1995 Jan 18;87(2):117–122. doi: 10.1093/jnci/87.2.117. [DOI] [PubMed] [Google Scholar]
- Young S. D., Hill R. P. Effects of reoxygenation on cells from hypoxic regions of solid tumors: anticancer drug sensitivity and metastatic potential. J Natl Cancer Inst. 1990 Mar 7;82(5):371–380. doi: 10.1093/jnci/82.5.371. [DOI] [PubMed] [Google Scholar]
- Young S. D., Marshall R. S., Hill R. P. Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9533–9537. doi: 10.1073/pnas.85.24.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]


