Abstract
Bacteriochlorin a (BCA) is a second-generation photosensitizer that is effective in tumour destruction upon illumination with light of a wavelength of 760 nm. Tissue penetration by light at this wavelength is greater compared with wavelengths at which commonly used photosensitizers are illuminated, making it possible to treat larger tumours. In a model of experimental liver metastases in rats, we measured lesion sizes after interstitial illumination of tumours at different times after intravenous administration of BCA (10 mg kg(-1) bodyweight), as well as BCA concentrations in liver and tumour tissue. In both, BCA concentrations showed a rapid decline within the first 4 h, followed by a slow decrease over the next 20 h, suggesting biphasic pharmacokinetics. No selective uptake in tumour tissue was observed. A near-linear relationship was found between lesion sizes and liver and tumour BCA concentrations, suggesting that optimal results with photodynamic therapy (PDT) could be obtained by illumination within a short time interval after administration, when tissue concentrations are highest. No severe liver toxicity was observed as indicated by serum ALAT levels. However, in all tumours evaluated, islands of vital-looking cells were present leading to tumour regrowth within 35 days. In view of the obtained lesion diameters of approximately 13 mm after BCA-PDT and the rapid clearance rate of BCA, the concept of a near-infrared absorbing photosensitizer for PDT of liver tumours is a potential interesting strategy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman N. B., Lien W. M., Kondi E. S., Silverman N. A. The blood supply of experimental liver metastases. I. The distribution of hepatic artery and portal vein blood to "small" and "large" tumors. Surgery. 1969 Dec;66(6):1067–1072. [PubMed] [Google Scholar]
- Arnfield M. R., Mathew R. P., Tulip J., McPhee M. S. Analysis of tissue optical coefficients using an approximate equation valid for comparable absorption and scattering. Phys Med Biol. 1992 Jun;37(6):1219–1230. doi: 10.1088/0031-9155/37/6/002. [DOI] [PubMed] [Google Scholar]
- Arnfield M., Gonzalez S., Lea P., Tulip J., McPhee M. Cylindrical irradiator fiber tip for photodynamic therapy. Lasers Surg Med. 1986;6(2):150–154. doi: 10.1002/lsm.1900060211. [DOI] [PubMed] [Google Scholar]
- Beems E. M., Dubbelman T. M., Lugtenburg J., Van Best J. A., Smeets M. F., Boegheim J. P. Photosensitizing properties of bacteriochlorophyllin a and bacteriochlorin a, two derivatives of bacteriochlorophyll a. Photochem Photobiol. 1987 Nov;46(5):639–643. doi: 10.1111/j.1751-1097.1987.tb04825.x. [DOI] [PubMed] [Google Scholar]
- Bellnier D. A., Dougherty T. J. The time course of cutaneous porphyrin photosensitization in the murine ear. Photochem Photobiol. 1989 Mar;49(3):369–372. doi: 10.1111/j.1751-1097.1989.tb04121.x. [DOI] [PubMed] [Google Scholar]
- Bellnier D. A., Ho Y. K., Pandey R. K., Missert J. R., Dougherty T. J. Distribution and elimination of Photofrin II in mice. Photochem Photobiol. 1989 Aug;50(2):221–228. doi: 10.1111/j.1751-1097.1989.tb04152.x. [DOI] [PubMed] [Google Scholar]
- Bown S. G., Tralau C. J., Smith P. D., Akdemir D., Wieman T. J. Photodynamic therapy with porphyrin and phthalocyanine sensitisation: quantitative studies in normal rat liver. Br J Cancer. 1986 Jul;54(1):43–52. doi: 10.1038/bjc.1986.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J. C., Adams G. E., Pearson J. K., Sansom J. M., Stratford I. J., Bedwell J., Bown S. G., MacRobert A. J., Phillips D. Increasing the effect of photodynamic therapy on the RIF-1 murine sarcoma, using the bioreductive drugs RSU1069 and RB6145. Br J Cancer. 1992 Dec;66(6):1070–1076. doi: 10.1038/bjc.1992.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugelski P. J., Porter C. W., Dougherty T. J. Autoradiographic distribution of hematoporphyrin derivative in normal and tumor tissue of the mouse. Cancer Res. 1981 Nov;41(11 Pt 1):4606–4612. [PubMed] [Google Scholar]
- Fingar V. H., Wieman T. J., Doak K. W. Role of thromboxane and prostacyclin release on photodynamic therapy-induced tumor destruction. Cancer Res. 1990 May 1;50(9):2599–2603. [PubMed] [Google Scholar]
- Fisher A. M., Murphree A. L., Gomer C. J. Clinical and preclinical photodynamic therapy. Lasers Surg Med. 1995;17(1):2–31. doi: 10.1002/lsm.1900170103. [DOI] [PubMed] [Google Scholar]
- Freitas I., Pontiggia P., Baronzio G. F., McLaren J. R. Perspectives for the combined use of photodynamic therapy and hyperthermia in cancer patient. Adv Exp Med Biol. 1990;267:511–520. doi: 10.1007/978-1-4684-5766-7_55. [DOI] [PubMed] [Google Scholar]
- Gomer C. J. Preclinical examination of first and second generation photosensitizers used in photodynamic therapy. Photochem Photobiol. 1991 Dec;54(6):1093–1107. doi: 10.1111/j.1751-1097.1991.tb02133.x. [DOI] [PubMed] [Google Scholar]
- Henderson B. W., Fingar V. H. Oxygen limitation of direct tumor cell kill during photodynamic treatment of a murine tumor model. Photochem Photobiol. 1989 Mar;49(3):299–304. doi: 10.1111/j.1751-1097.1989.tb04110.x. [DOI] [PubMed] [Google Scholar]
- Henderson B. W., Sumlin A. B., Owczarczak B. L., Dougherty T. J. Bacteriochlorophyll-a as photosensitizer for photodynamic treatment of transplantable murine tumors. J Photochem Photobiol B. 1991 Sep;10(4):303–313. doi: 10.1016/1011-1344(91)80016-b. [DOI] [PubMed] [Google Scholar]
- Holt S., Tulip J., Hamilton D., Cummins J., Fields A., Dick C. Experimental laser phototherapy of the Morris 7777 hepatoma in the rat. Hepatology. 1985 Mar-Apr;5(2):175–180. doi: 10.1002/hep.1840050203. [DOI] [PubMed] [Google Scholar]
- Korbelik M., Krosl G. Cellular levels of photosensitisers in tumours: the role of proximity to the blood supply. Br J Cancer. 1994 Oct;70(4):604–610. doi: 10.1038/bjc.1994.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marijnissen J. P., Versteeg J. A., Star W. M., van Putten W. L. Tumor and normal tissue response to interstitial photodynamic therapy of the rat R-1 rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 1992;22(5):963–972. doi: 10.1016/0360-3016(92)90795-j. [DOI] [PubMed] [Google Scholar]
- Nishiwaki Y., Nakamura S., Sakaguchi S. New method of photosensitizer accumulation for photodynamic therapy in an experimental liver tumor. Lasers Surg Med. 1989;9(3):254–263. doi: 10.1002/lsm.1900090308. [DOI] [PubMed] [Google Scholar]
- Schuitmaker J. J., van Best J. A., van Delft J. L., Dubbelman T. M., Oosterhuis J. A., de Wolff-Rouendaal D. Bacteriochlorin a, a new photosensitizer in photodynamic therapy. In vivo results. Invest Ophthalmol Vis Sci. 1990 Aug;31(8):1444–1450. [PubMed] [Google Scholar]
- Svanberg K., Liu D. L., Wang I., Andersson-Engels S., Stenram U., Svanberg S. Photodynamic therapy using intravenous delta-aminolaevulinic acid-induced protoporphyrin IX sensitisation in experimental hepatic tumours in rats. Br J Cancer. 1996 Nov;74(10):1526–1533. doi: 10.1038/bjc.1996.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldow S. M., Henderson B. W., Dougherty T. J. Potentiation of photodynamic therapy by heat: effect of sequence and time interval between treatments in vivo. Lasers Surg Med. 1985;5(2):83–94. doi: 10.1002/lsm.1900050203. [DOI] [PubMed] [Google Scholar]
- Wilson B. C., Patterson M. S. The physics of photodynamic therapy. Phys Med Biol. 1986 Apr;31(4):327–360. doi: 10.1088/0031-9155/31/4/001. [DOI] [PubMed] [Google Scholar]
- de Smidt P. C., Versluis A. J., van Berkel T. J. Transport of sulfonated tetraphenylporphine by lipoproteins in the hamster. Biochem Pharmacol. 1992 Jun 23;43(12):2567–2573. doi: 10.1016/0006-2952(92)90145-9. [DOI] [PubMed] [Google Scholar]
- van Geel I. P., Oppelaar H., Oussoren Y. G., Schuitmaker J. J., Stewart F. A. Mechanisms for optimising photodynamic therapy: second-generation photosensitisers in combination with mitomycin C. Br J Cancer. 1995 Aug;72(2):344–350. doi: 10.1038/bjc.1995.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
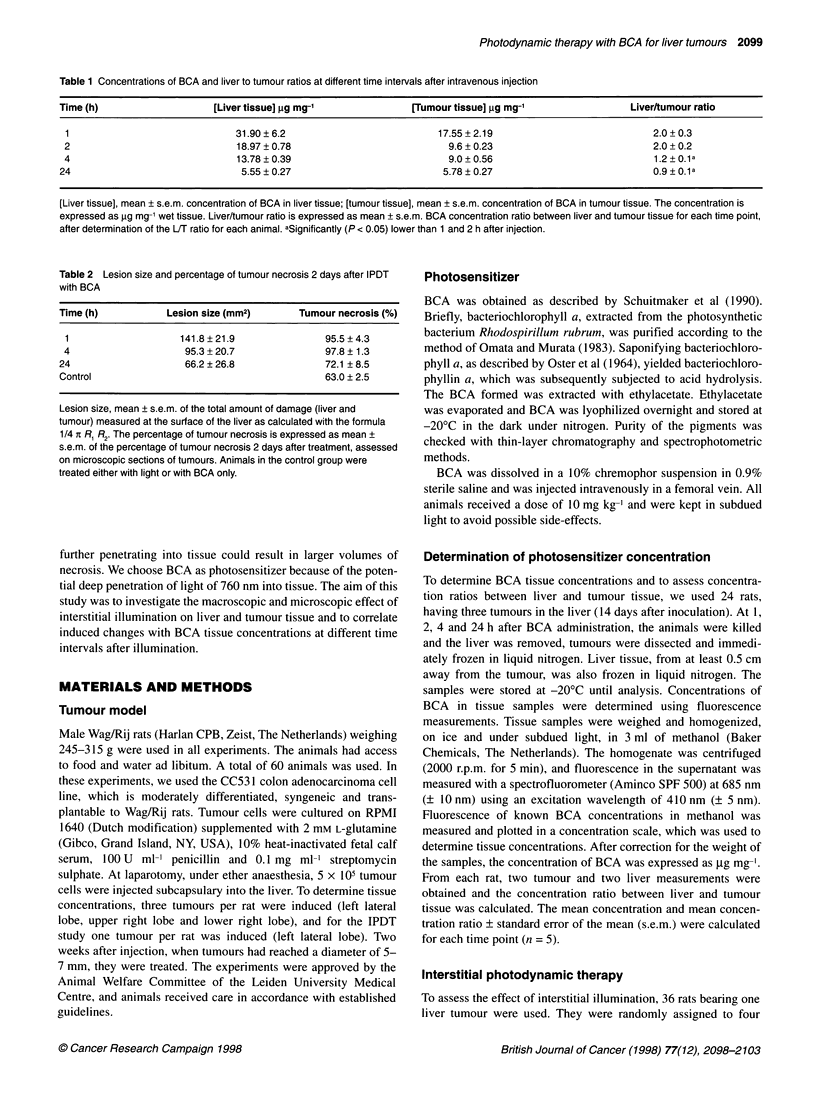
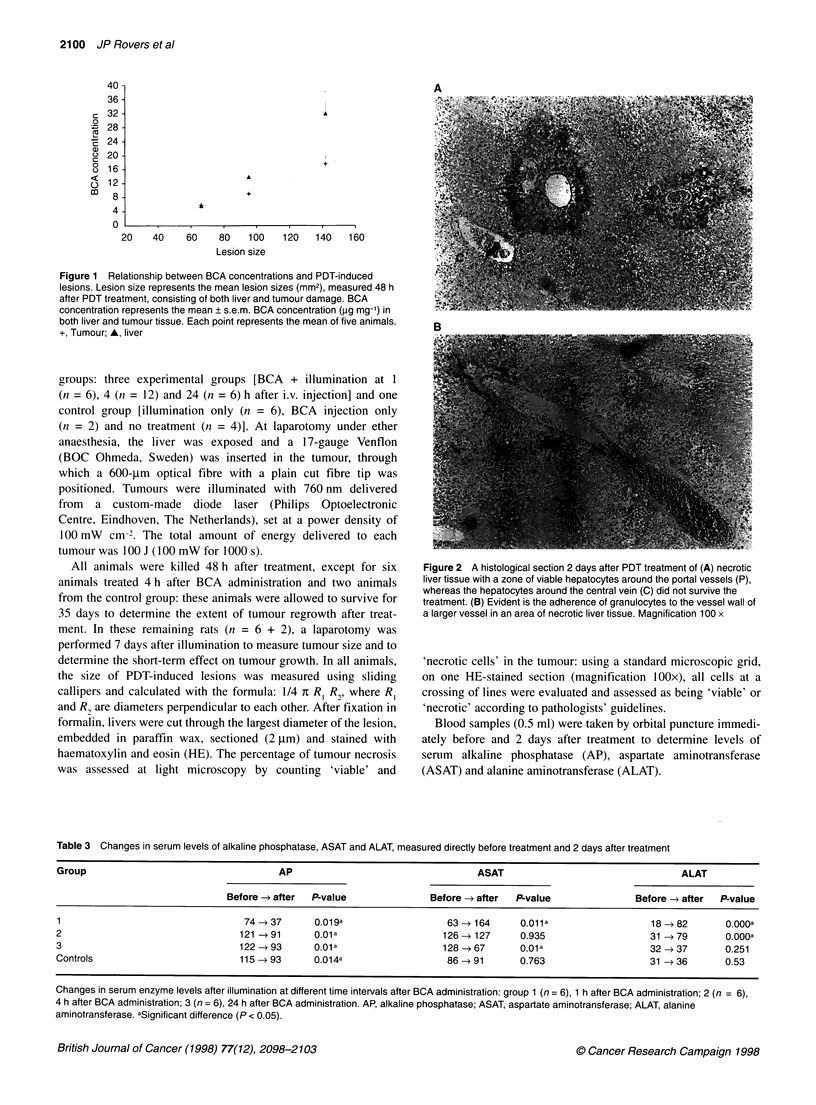
- van Hillegersberg R., Marijnissen J. P., Kort W. J., Zondervan P. E., Terpstra O. T., Star W. M. Interstitial photodynamic therapy in a rat liver metastasis model. Br J Cancer. 1992 Dec;66(6):1005–1014. doi: 10.1038/bjc.1992.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leengoed H. L., Schuitmaker J. J., van der Veen N., Dubbelman T. M., Star W. M. Fluorescence and photodynamic effects of bacteriochlorin a observed in vivo in 'sandwich' observation chambers. Br J Cancer. 1993 May;67(5):898–903. doi: 10.1038/bjc.1993.168. [DOI] [PMC free article] [PubMed] [Google Scholar]