Abstract
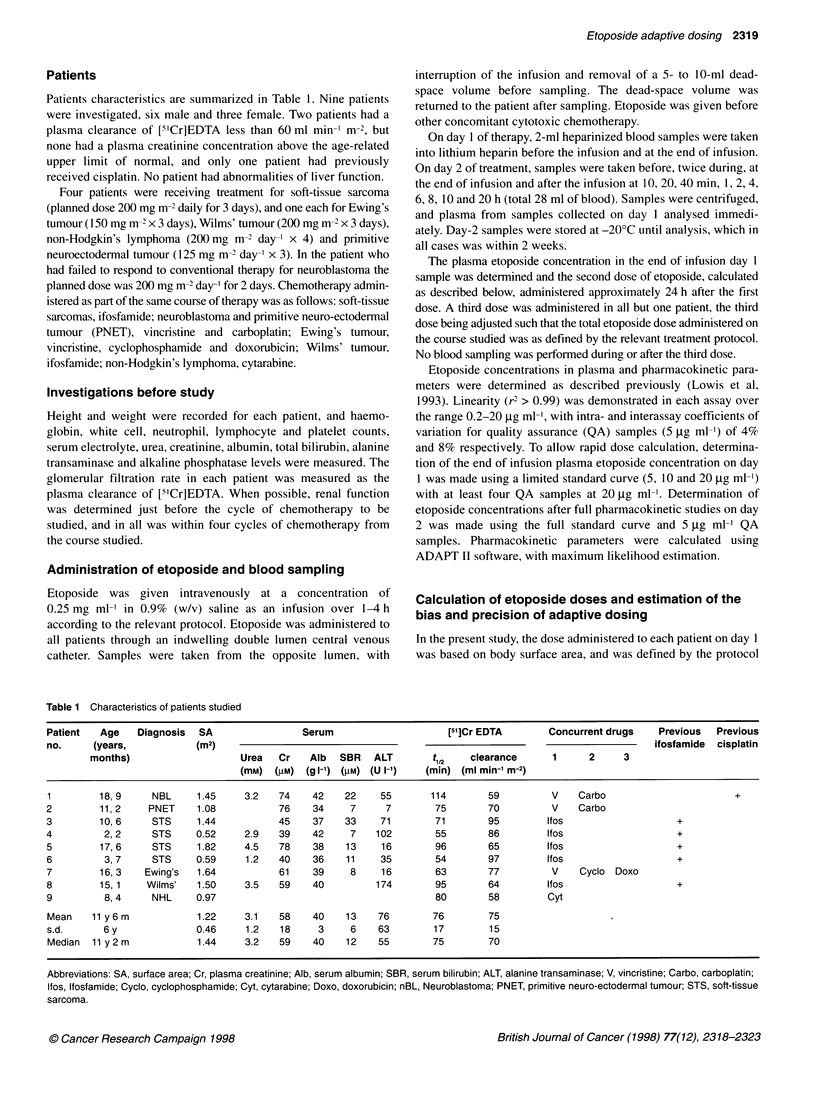
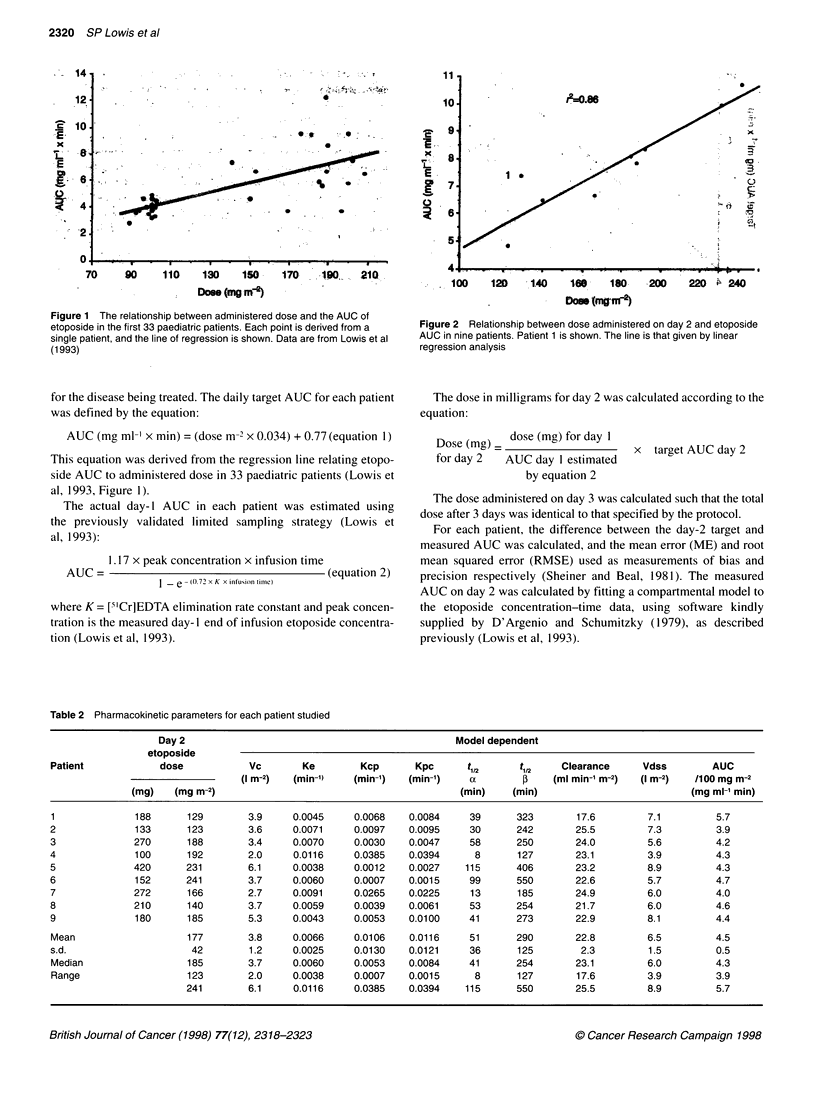
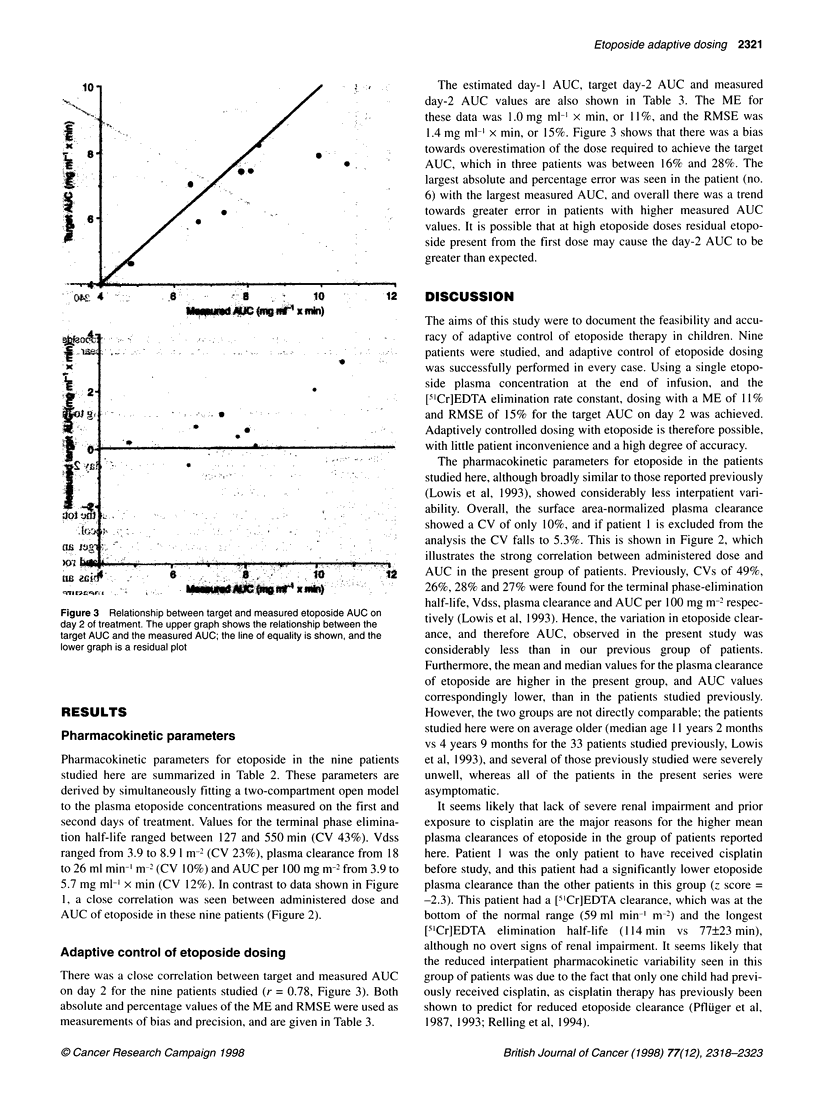
Pharmacokinetically guided dosing was performed in nine paediatric patients receiving etoposide. Doses on day 2 of a 2- or 3-day schedule were adapted on the basis of the day-1 area under the plasma etoposide concentration vs time curve (AUC). The day-1 AUC was estimated using a limited sampling model and the day-2 target AUC defined by the etoposide dose-AUC relationship observed in 33 children. Target AUC values (4.6-8.2 mg ml(-1) x min) were achieved with a high degree of precision and with little bias (mean error 11% and root mean squared error 15% respectively). Pharmacokinetic parameters were similar to those reported previously in children, although interpatient pharmacokinetic variability was less than that observed previously: plasma clearance, 23 (18-26) ml min(-1) m(-2); volume of distribution at steady state (Vdss), 6.0 (3.9-8.9) l m(-2); t(1/2) 254 (127-550) min (median and range). This study has demonstrated that pharmacokinetically guided dosing with etoposide is feasible. However, pharmacokinetically guided dosing is likely to be of most benefit in patients with abnormalities of renal or hepatic function, or in children with prior exposure to cisplatin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisner J., Van Echo D. A., Whitacre M., Wiernik P. H. A phase I trial of continuous infusion VP16-213 (etoposide). Cancer Chemother Pharmacol. 1982;7(2-3):157–160. doi: 10.1007/BF00254539. [DOI] [PubMed] [Google Scholar]
- Bennett C. L., Sinkule J. A., Schilsky R. L., Senekjian E., Choi K. E. Phase I clinical and pharmacological study of 72-hour continuous infusion of etoposide in patients with advanced cancer. Cancer Res. 1987 Apr 1;47(7):1952–1956. [PubMed] [Google Scholar]
- Boos J., Krümpelmann S., Schulze-Westhoff P., Euting T., Berthold F., Jürgens H. Steady-state levels and bone marrow toxicity of etoposide in children and infants: does etoposide require age-dependent dose calculation? J Clin Oncol. 1995 Dec;13(12):2954–2960. doi: 10.1200/JCO.1995.13.12.2954. [DOI] [PubMed] [Google Scholar]
- D'Argenio D. Z., Schumitzky A. A program package for simulation and parameter estimation in pharmacokinetic systems. Comput Programs Biomed. 1979 Mar;9(2):115–134. doi: 10.1016/0010-468x(79)90025-4. [DOI] [PubMed] [Google Scholar]
- English M. W., Lowis S. P., Peng B., Boddy A., Newell D. R., Price L., Pearson A. D. Pharmacokinetically guided dosing of carboplatin and etoposide during peritoneal dialysis and haemodialysis. Br J Cancer. 1996 Mar;73(6):776–780. doi: 10.1038/bjc.1996.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka M., Masuda N., Negoro S., Takada M., Kudoh S., Kusunoki Y., Matsui K., Takifuji N., Tachikawa A., Kawahara M. A phase I study of chronic daily dosing of oral etoposide in combination with cisplatin for patients with advanced cancer. Cancer. 1991 Jul 15;68(2):284–288. doi: 10.1002/1097-0142(19910715)68:2<284::aid-cncr2820680212>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Gentili D., Zucchetti M., Torri V., Sessa C., de Jong J., Cavalli F., D'Incalci M. A limited sampling model for the pharmacokinetics of etoposide given orally. Cancer Chemother Pharmacol. 1993;32(6):482–486. doi: 10.1007/BF00685894. [DOI] [PubMed] [Google Scholar]
- Henwood J. M., Brogden R. N. Etoposide. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in combination chemotherapy of cancer. Drugs. 1990 Mar;39(3):438–490. doi: 10.2165/00003495-199039030-00008. [DOI] [PubMed] [Google Scholar]
- Joel S. P., Ellis P., O'Byrne K., Papamichael D., Hall M., Penson R., Nicholls S., O'Donnell C., Constantinou A., Woodhull J. Therapeutic monitoring of continuous infusion etoposide in small-cell lung cancer. J Clin Oncol. 1996 Jun;14(6):1903–1912. doi: 10.1200/JCO.1996.14.6.1903. [DOI] [PubMed] [Google Scholar]
- Kunitoh H., Watanabe K. Phase I/II and pharmacologic study of long-term continuous infusion etoposide combined with cisplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol. 1994 Jan;12(1):83–89. doi: 10.1200/JCO.1994.12.1.83. [DOI] [PubMed] [Google Scholar]
- Lokich J., Anderson N., Bern M., Wallach S., Moore C., Williams D. Etoposide admixed with cisplatin. Phase I clinical investigation of 72-hour infusion. Cancer. 1989 Mar 1;63(5):818–821. doi: 10.1002/1097-0142(19890301)63:5<818::aid-cncr2820630503>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Lokich J., Corkery J. Phase I study of VP-16-213 (Etoposide) administered as a continuous 5-day infusion. Cancer Treat Rep. 1981 Sep-Oct;65(9-10):887–889. [PubMed] [Google Scholar]
- Lowis S. P., Pearson A. D., Newell D. R., Cole M. Etoposide pharmacokinetics in children: the development and prospective validation of a dosing equation. Cancer Res. 1993 Oct 15;53(20):4881–4889. [PubMed] [Google Scholar]
- Miller A. A., Tolley E. A. Predictive performance of a pharmacodynamic model for oral etoposide. Cancer Res. 1994 Apr 15;54(8):2080–2083. [PubMed] [Google Scholar]
- Minami H., Shimokata K., Saka H., Saito H., Ando Y., Senda K., Nomura F., Sakai S. Phase I clinical and pharmacokinetic study of a 14-day infusion of etoposide in patients with lung cancer. J Clin Oncol. 1993 Aug;11(8):1602–1608. doi: 10.1200/JCO.1993.11.8.1602. [DOI] [PubMed] [Google Scholar]
- Pflüger K. H., Hahn M., Holz J. B., Schmidt L., Köhl P., Fritsch H. W., Jungclas H., Havemann K. Pharmacokinetics of etoposide: correlation of pharmacokinetic parameters with clinical conditions. Cancer Chemother Pharmacol. 1993;31(5):350–356. doi: 10.1007/BF00686147. [DOI] [PubMed] [Google Scholar]
- Pflüger K. H., Schmidt L., Merkel M., Jungclas H., Havemann K. Drug monitoring of etoposide (VP16-213). Correlation of pharmacokinetic parameters to clinical and biochemical data from patients receiving etoposide. Cancer Chemother Pharmacol. 1987;20(1):59–66. doi: 10.1007/BF00252961. [DOI] [PubMed] [Google Scholar]
- Ratain M. J., Mick R., Schilsky R. L., Vogelzang N. J., Berezin F. Pharmacologically based dosing of etoposide: a means of safely increasing dose intensity. J Clin Oncol. 1991 Aug;9(8):1480–1486. doi: 10.1200/JCO.1991.9.8.1480. [DOI] [PubMed] [Google Scholar]
- Ratain M. J., Schilsky R. L., Choi K. E., Guarnieri C., Grimmer D., Vogelzang N. J., Senekjian E., Liebner M. A. Adaptive control of etoposide administration: impact of interpatient pharmacodynamic variability. Clin Pharmacol Ther. 1989 Mar;45(3):226–233. doi: 10.1038/clpt.1989.22. [DOI] [PubMed] [Google Scholar]
- Relling M. V., McLeod H. L., Bowman L. C., Santana V. M. Etoposide pharmacokinetics and pharmacodynamics after acute and chronic exposure to cisplatin. Clin Pharmacol Ther. 1994 Nov;56(5):503–511. doi: 10.1038/clpt.1994.171. [DOI] [PubMed] [Google Scholar]
- Sheiner L. B., Beal S. L. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981 Aug;9(4):503–512. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- Strömgren A. S., Sørensen B. T., Jakobsen P., Jakobsen A. A limited sampling method for estimation of the etoposide area under the curve. Cancer Chemother Pharmacol. 1993;32(3):226–230. doi: 10.1007/BF00685840. [DOI] [PubMed] [Google Scholar]


