Abstract
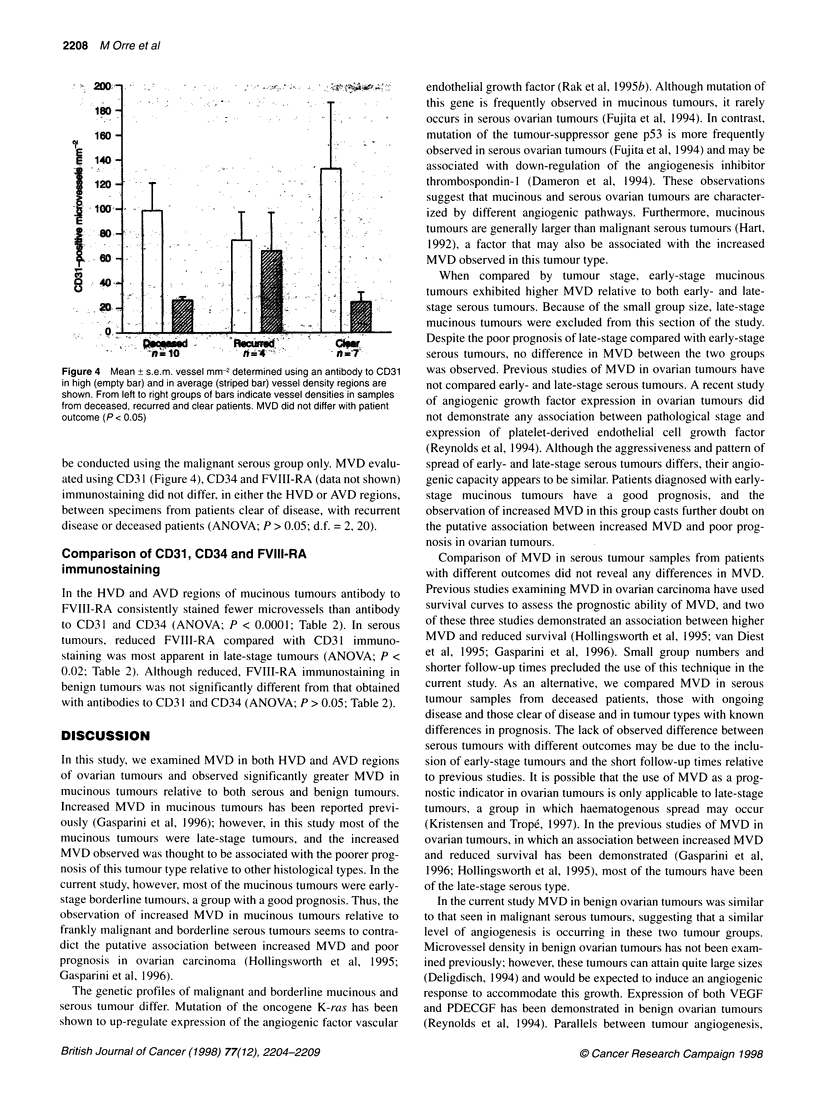
Microvessel density of benign, borderline and malignant ovarian tumours was studied immunohistochemically using antibodies to the endothelial cell markers CD31, CD34 and factor VIII-related antigen. Microvessel density was compared in tumours of different histological subtype, stage and patient outcome. CD31-immunostained sections were examined and regions of high and average microvessel density were selected. Identical regions were located on CD34- and factor VIII-related antigen-immunostained serial sections and microvessel counts obtained and converted to vessels mm(-2). CD31 and CD34 immunostaining revealed increased microvessel density in both the high and average vessel density regions of mucinous (222.4 +/- 24.8; 79.9 +/- 8.5) compared with serous (105.4 +/- 20.7; 33.3 +/- 6.8) and benign (84.4 +/- 19.4; 20.4 +/- 4.4) tumours (P < 0.001). CD31 and CD34 immunostaining also revealed increased microvessel density in early-stage mucinous tumours (234.6 +/- 28.2; 87.8 +/- 9.2) compared with that observed in both early- (72.8 +/- 15; 12.9 +/- 2.4) and late- (115.6 +/- 26.5; 29.8 +/- 8.5) stage serous tumours (P < 0.001). No differences in microvessel density in samples from patients with differing outcomes were observed (P > 0.05). Reduced factor VIII-related antigen compared with CD31 and CD34 immunostaining was observed in both borderline and malignant mucinous and serous tumours (P < 0.02) but not in benign tumours (P > 0.05). Our results contradict the putative association between increased microvessel density and poor prognosis and suggest that the level and control of angiogenesis may differ between ovarian tumour types.
Full text
PDF
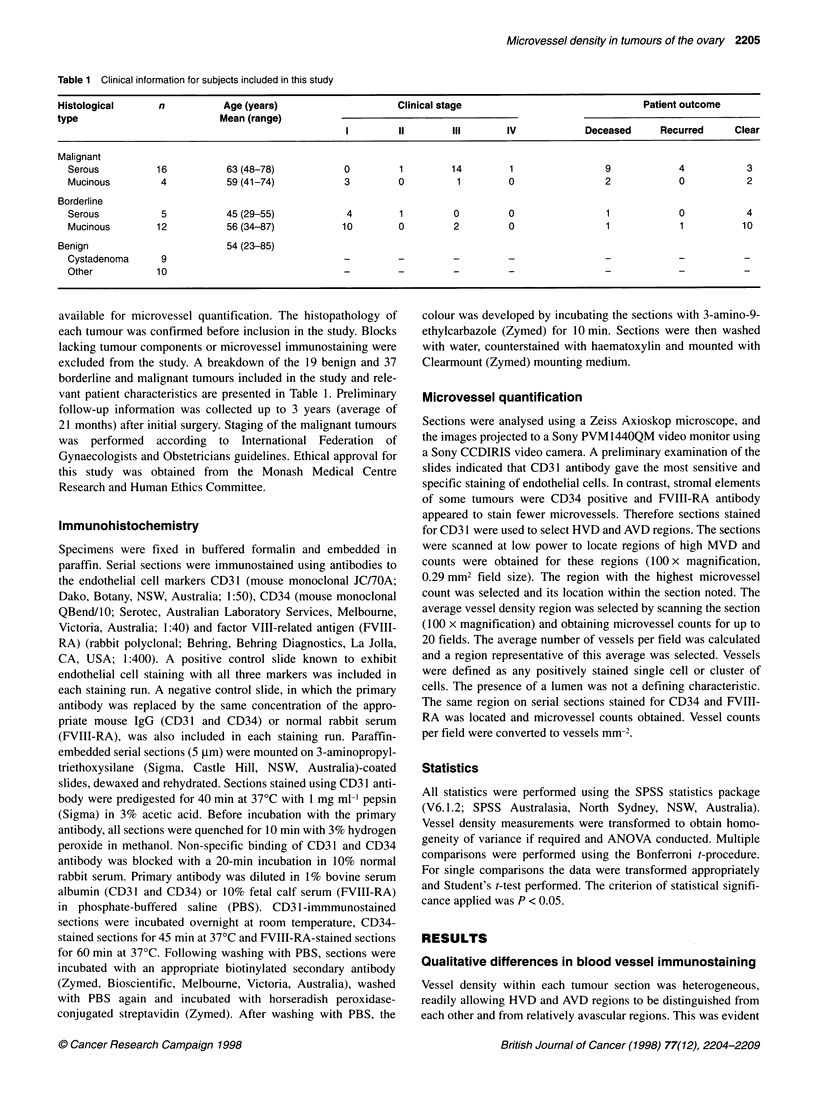
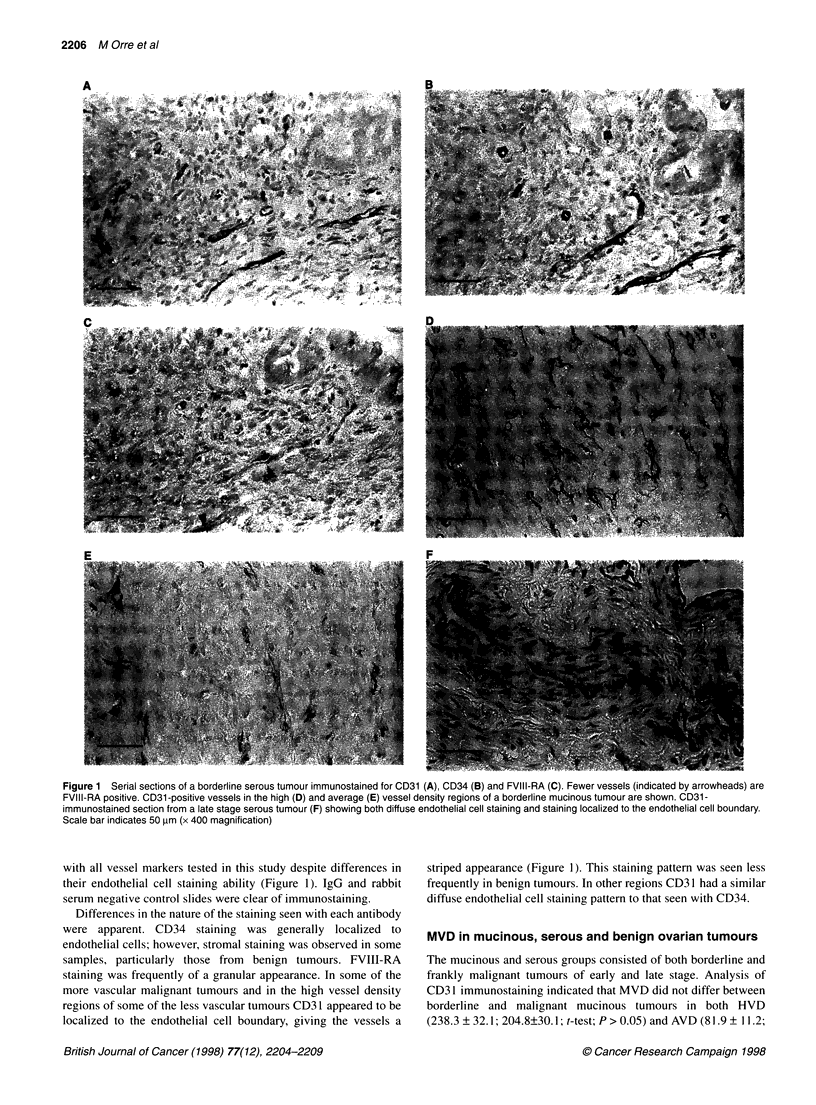
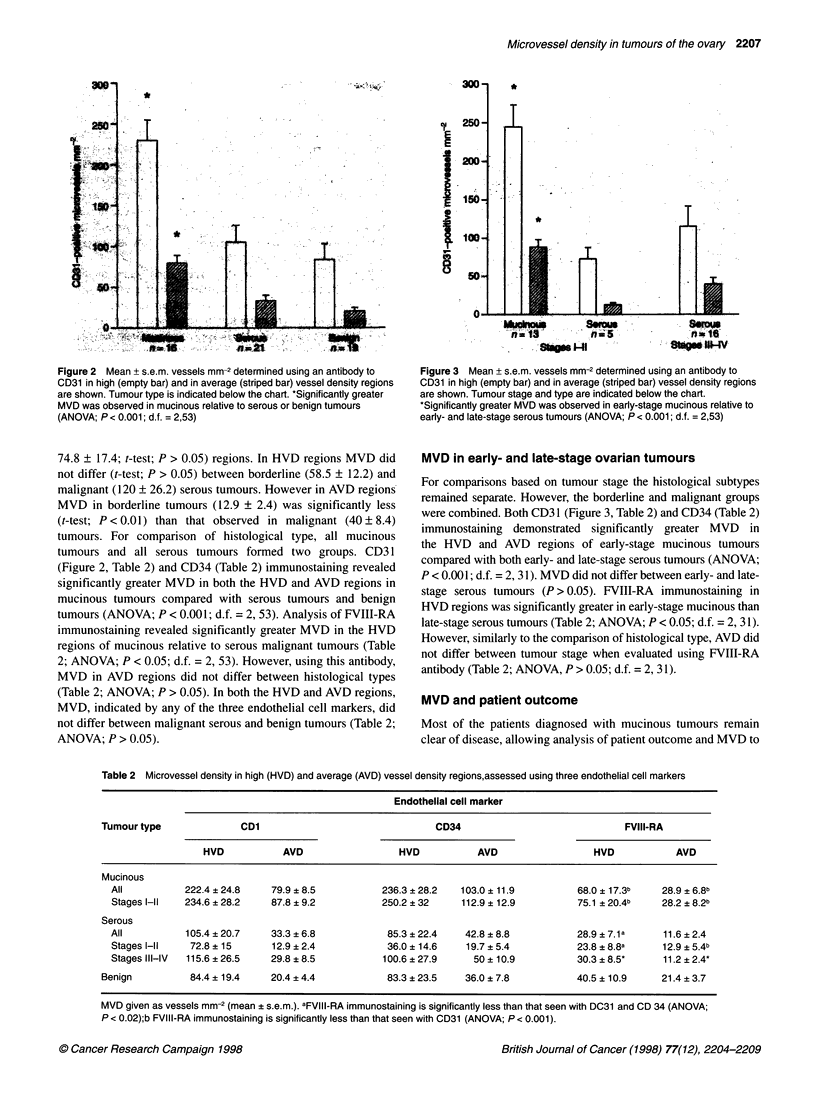

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaubal A., Paetau A., Zoltick P., Miettinen M. CD34 immunoreactivity in nervous system tumors. Acta Neuropathol. 1994;88(5):454–458. doi: 10.1007/BF00389498. [DOI] [PubMed] [Google Scholar]
- Dameron K. M., Volpert O. V., Tainsky M. A., Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994 Sep 9;265(5178):1582–1584. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- DeLisser H. M., Newman P. J., Albelda S. M. Molecular and functional aspects of PECAM-1/CD31. Immunol Today. 1994 Oct;15(10):490–495. doi: 10.1016/0167-5699(94)90195-3. [DOI] [PubMed] [Google Scholar]
- Fay P. J. Factor VIII structure and function. Thromb Haemost. 1993 Jul 1;70(1):63–67. [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- Fujita M., Enomoto T., Inoue M., Tanizawa O., Ozaki M., Rice J. M., Nomura T. Alteration of the p53 tumor suppressor gene occurs independently of K-ras activation and more frequently in serous adenocarcinomas than in other common epithelial tumors of the human ovary. Jpn J Cancer Res. 1994 Dec;85(12):1247–1256. doi: 10.1111/j.1349-7006.1994.tb02937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini G., Bonoldi E., Viale G., Verderio P., Boracchi P., Panizzoni G. A., Radaelli U., Di Bacco A., Guglielmi R. B., Bevilacqua P. Prognostic and predictive value of tumour angiogenesis in ovarian carcinomas. Int J Cancer. 1996 Jun 21;69(3):205–211. doi: 10.1002/(SICI)1097-0215(19960621)69:3<205::AID-IJC10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Hollingsworth H. C., Kohn E. C., Steinberg S. M., Rothenberg M. L., Merino M. J. Tumor angiogenesis in advanced stage ovarian carcinoma. Am J Pathol. 1995 Jul;147(1):33–41. [PMC free article] [PubMed] [Google Scholar]
- Krause D. S., Fackler M. J., Civin C. I., May W. S. CD34: structure, biology, and clinical utility. Blood. 1996 Jan 1;87(1):1–13. [PubMed] [Google Scholar]
- Kristensen G. B., Tropé C. Epithelial ovarian carcinoma. Lancet. 1997 Jan 11;349(9045):113–117. doi: 10.1016/S0140-6736(96)06071-0. [DOI] [PubMed] [Google Scholar]
- Kuzu I., Bicknell R., Harris A. L., Jones M., Gatter K. C., Mason D. Y. Heterogeneity of vascular endothelial cells with relevance to diagnosis of vascular tumours. J Clin Pathol. 1992 Feb;45(2):143–148. doi: 10.1136/jcp.45.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Kleinerman J., Saidel G. M. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res. 1974 May;34(5):997–1004. [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- McCulloch P., Choy A., Martin L. Association between tumour angiogenesis and tumour cell shedding into effluent venous blood during breast cancer surgery. Lancet. 1995 Nov 18;346(8986):1334–1335. doi: 10.1016/s0140-6736(95)92345-4. [DOI] [PubMed] [Google Scholar]
- Rak J. W., St Croix B. D., Kerbel R. S. Consequences of angiogenesis for tumor progression, metastasis and cancer therapy. Anticancer Drugs. 1995 Feb;6(1):3–18. doi: 10.1097/00001813-199502000-00001. [DOI] [PubMed] [Google Scholar]
- Rak J., Mitsuhashi Y., Bayko L., Filmus J., Shirasawa S., Sasazuki T., Kerbel R. S. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995 Oct 15;55(20):4575–4580. [PubMed] [Google Scholar]
- Reynolds K., Farzaneh F., Collins W. P., Campbell S., Bourne T. H., Lawton F., Moghaddam A., Harris A. L., Bicknell R. Association of ovarian malignancy with expression of platelet-derived endothelial cell growth factor. J Natl Cancer Inst. 1994 Aug 17;86(16):1234–1238. doi: 10.1093/jnci/86.16.1234. [DOI] [PubMed] [Google Scholar]
- Schlingemann R. O., Rietveld F. J., Kwaspen F., van de Kerkhof P. C., de Waal R. M., Ruiter D. J. Differential expression of markers for endothelial cells, pericytes, and basal lamina in the microvasculature of tumors and granulation tissue. Am J Pathol. 1991 Jun;138(6):1335–1347. [PMC free article] [PubMed] [Google Scholar]
- Sheid B. Angiogenic effects of macrophages isolated from ascitic fluid aspirated from women with advanced ovarian cancer. Cancer Lett. 1992 Feb 29;62(2):153–158. doi: 10.1016/0304-3835(92)90186-y. [DOI] [PubMed] [Google Scholar]
- Toi M., Kashitani J., Tominaga T. Tumor angiogenesis is an independent prognostic indicator in primary breast carcinoma. Int J Cancer. 1993 Sep 30;55(3):371–374. doi: 10.1002/ijc.2910550305. [DOI] [PubMed] [Google Scholar]
- Visscher D. W., Lawrence W. D., Boman S. Angiogenesis in breast carcinoma--clinicopathologic relevance and potential use as a quantifiable surrogate endpoint biomarker. J Cell Biochem Suppl. 1994;19:146–152. [PubMed] [Google Scholar]
- Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995 Jul;147(1):9–19. [PMC free article] [PubMed] [Google Scholar]
- van Diest P. J., Zevering J. P., Zevering L. C., Baak J. P. Prognostic value of microvessel quantitation in cisplatin treated FIGO 3 and 4 ovarian cancer patients. Pathol Res Pract. 1995 Feb;191(1):25–30. doi: 10.1016/S0344-0338(11)80918-0. [DOI] [PubMed] [Google Scholar]