Abstract
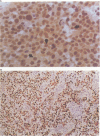
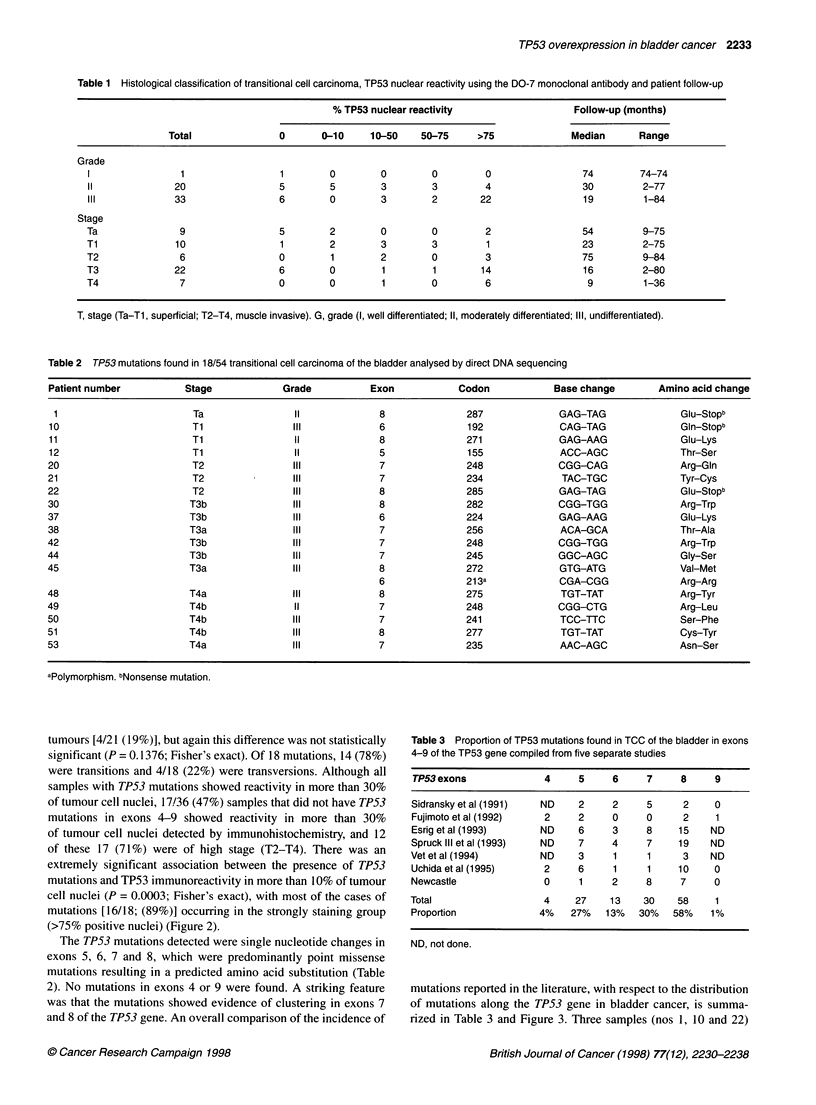
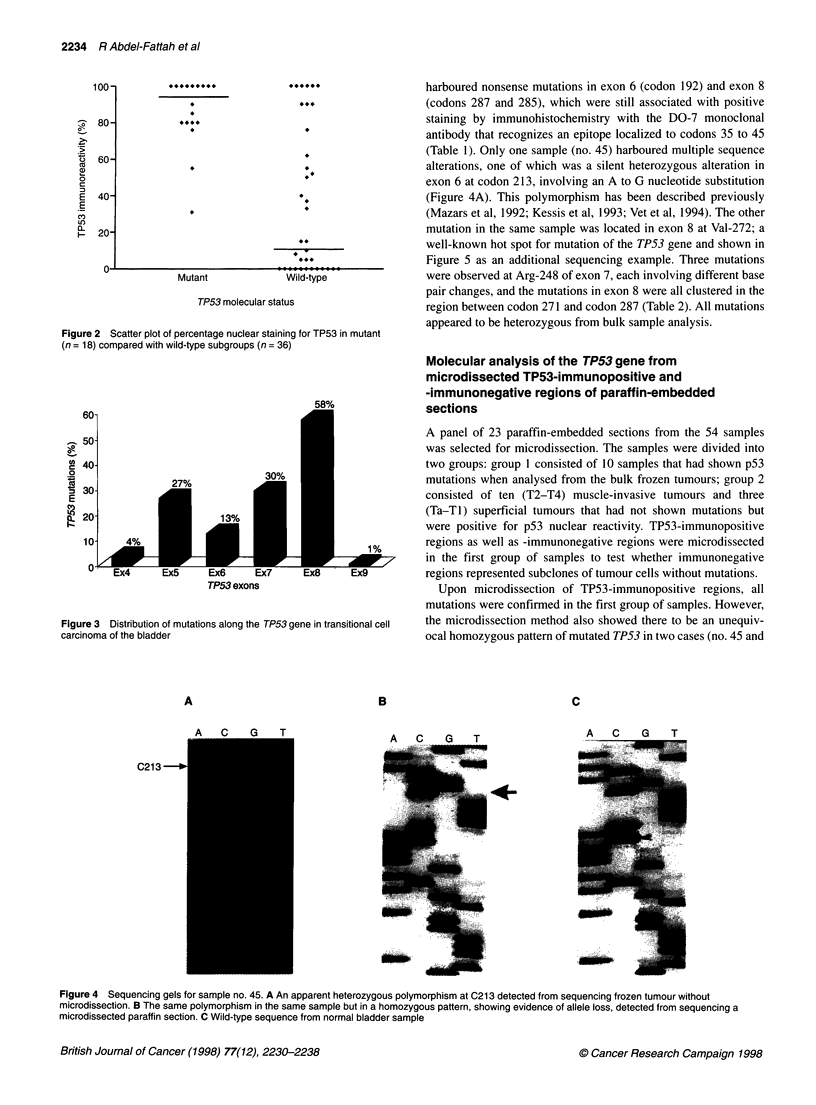
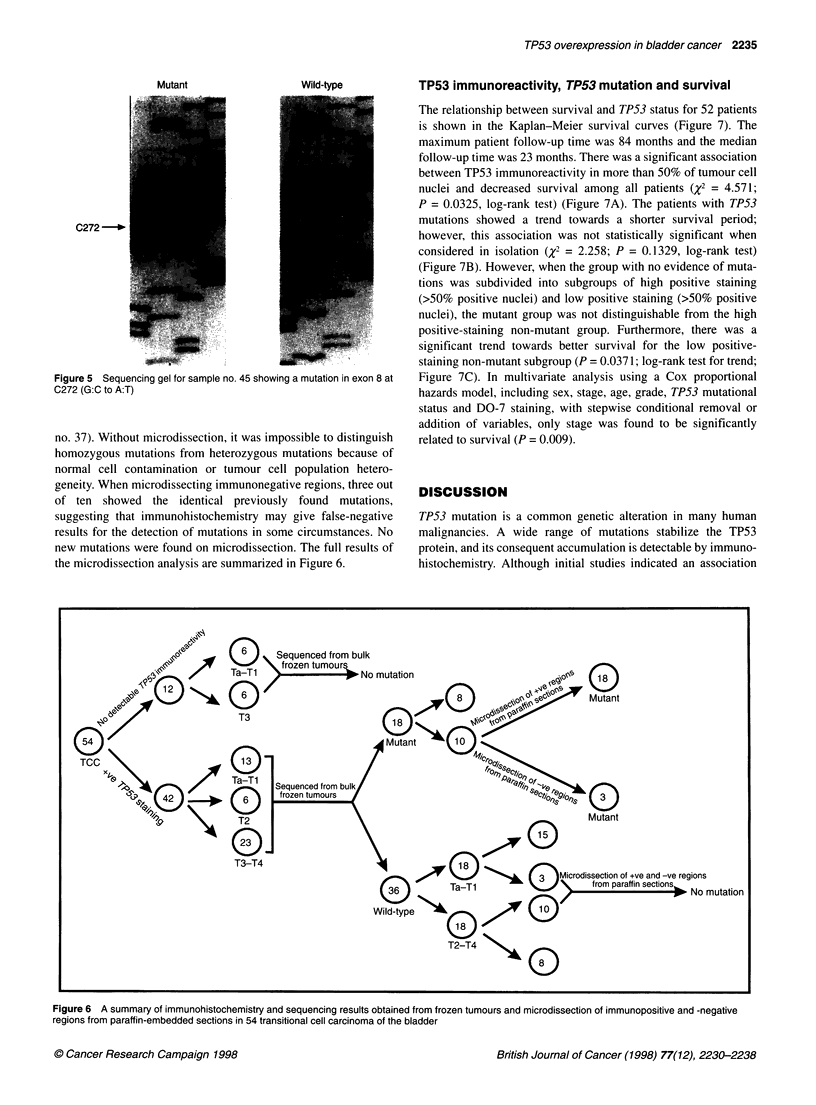
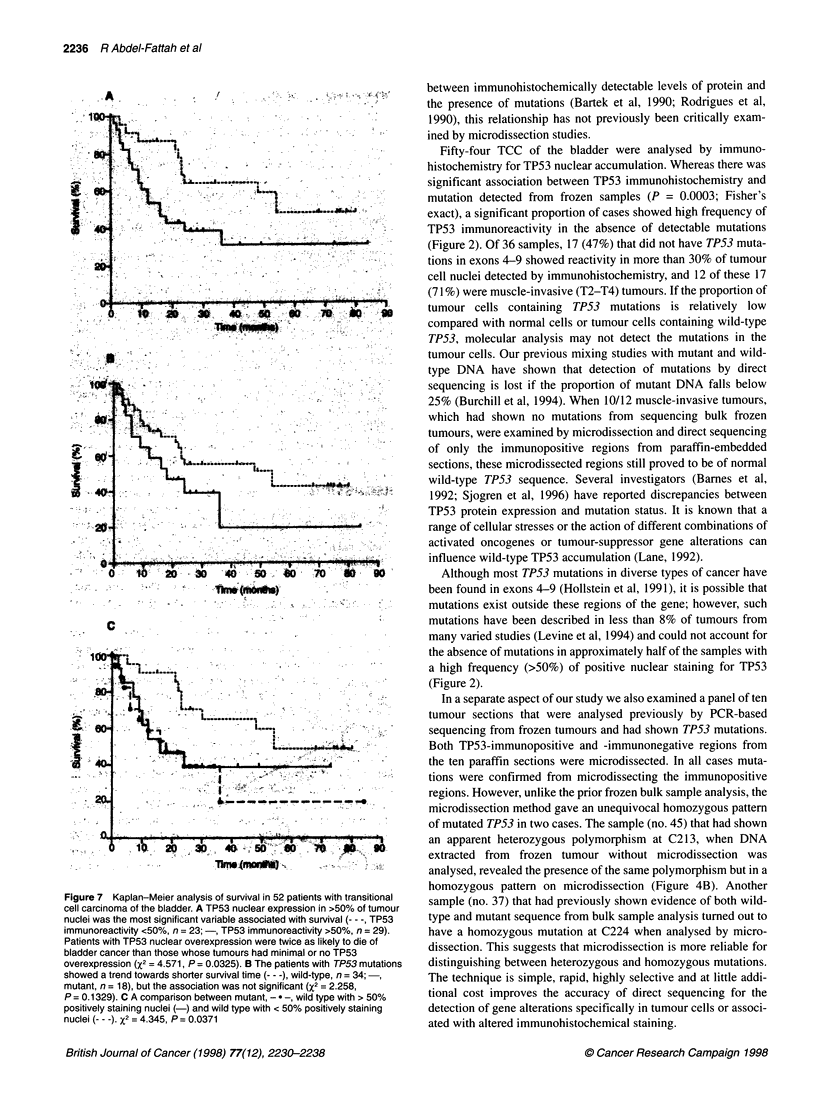
We have used microdissection of paraffin-embedded histological sections and polymerase chain reaction (PCR)-based direct DNA sequencing for 54 transitional cell carcinoma (TCC) of the bladder, to examine critically the association between TP53 nuclear accumulation determined by immunohistochemistry and the presence of TP53 mutations, and to examine their relationship to tumour stage and grade, as well as patient survival. There was a significant association between the presence of TP53-positive nuclei (> 10%) and a higher histological stage and grade (P = 0.0115, P = 0.0151 respectively; Fisher's exact). A significant association between TP53 gene mutations and TP53 nuclear reactivity in more than 10% of tumour cell nuclei was also observed (P = 0.0003; Fisher's exact). Mutations were detected in 18/54 (33%) cases together with the wild-type sequence when analysed from bulk frozen samples, with significant clustering of mutations in exons 7 and 8. The microdissection method distinguished more clearly between heterozygous and/or homozygous alterations of the TP53 tumour-suppressor gene, and clearly showed frequent accumulation of TP53 in the absence of mutations. When microdissecting immunonegative regions from the same paraffin sections, three out of ten samples showed the identical mutations detected in the immunopositive regions. There was a significant association between TP53 immunoreactivity in more than 50% of tumour cell nuclei and decreased survival among all patients (P = 0.0325; log-rank test). The patients with TP53 mutations showed a trend for a shorter survival period; however, the association was not statistically significant at the 95% confidence level (P = 0.132; log-rank test). In conclusion, our observations show that accumulation of TP53 occurs frequently in the absence of mutations, and that such accumulation is nevertheless associated with poor survival when it occurs in a high proportion (> 50%) of tumour cell nuclei.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker S. J., Preisinger A. C., Jessup J. M., Paraskeva C., Markowitz S., Willson J. K., Hamilton S., Vogelstein B. p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990 Dec 1;50(23):7717–7722. [PubMed] [Google Scholar]
- Barnes D. M., Hanby A. M., Gillett C. E., Mohammed S., Hodgson S., Bobrow L. G., Leigh I. M., Purkis T., MacGeoch C., Spurr N. K. Abnormal expression of wild type p53 protein in normal cells of a cancer family patient. Lancet. 1992 Aug 1;340(8814):259–263. doi: 10.1016/0140-6736(92)92354-i. [DOI] [PubMed] [Google Scholar]
- Burchill S. A., Neal D. E., Lunec J. Frequency of H-ras mutations in human bladder cancer detected by direct sequencing. Br J Urol. 1994 May;73(5):516–521. doi: 10.1111/j.1464-410x.1994.tb07636.x. [DOI] [PubMed] [Google Scholar]
- Bártek J., Bártková J., Vojtesek B., Stasková Z., Rejthar A., Kovarík J., Lane D. P. Patterns of expression of the p53 tumour suppressor in human breast tissues and tumours in situ and in vitro. Int J Cancer. 1990 Nov 15;46(5):839–844. doi: 10.1002/ijc.2910460515. [DOI] [PubMed] [Google Scholar]
- Challen C., Lunec J., Warren W., Collier J., Bassendine M. F. Analysis of the p53 tumor-suppressor gene in hepatocellular carcinomas from Britain. Hepatology. 1992 Dec;16(6):1362–1366. doi: 10.1002/hep.1840160610. [DOI] [PubMed] [Google Scholar]
- Chiba I., Takahashi T., Nau M. M., D'Amico D., Curiel D. T., Mitsudomi T., Buchhagen D. L., Carbone D., Piantadosi S., Koga H. Mutations in the p53 gene are frequent in primary, resected non-small cell lung cancer. Lung Cancer Study Group. Oncogene. 1990 Oct;5(10):1603–1610. [PubMed] [Google Scholar]
- Cho Y., Gorina S., Jeffrey P. D., Pavletich N. P. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science. 1994 Jul 15;265(5170):346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- Ellison D. W., Lunec J., Gallagher P. J., Steart P. V., Jaros E., Gatter K. C. Accumulation of wild-type p53 in meningiomas. Neuropathol Appl Neurobiol. 1995 Apr;21(2):136–142. doi: 10.1111/j.1365-2990.1995.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Esrig D., Spruck C. H., 3rd, Nichols P. W., Chaiwun B., Steven K., Groshen S., Chen S. C., Skinner D. G., Jones P. A., Cote R. J. p53 nuclear protein accumulation correlates with mutations in the p53 gene, tumor grade, and stage in bladder cancer. Am J Pathol. 1993 Nov;143(5):1389–1397. [PMC free article] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Tan T. H., Eliyahu D., Oren M., Levine A. J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988 Feb;8(2):531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K., Yamada Y., Okajima E., Kakizoe T., Sasaki H., Sugimura T., Terada M. Frequent association of p53 gene mutation in invasive bladder cancer. Cancer Res. 1992 Mar 15;52(6):1393–1398. [PubMed] [Google Scholar]
- Fujita J., Nakayama H., Onoue H., Rhim J. S., el-Bolkainy M. N., el-Aaser A. A., Kitamura Y. Frequency of active ras oncogenes in human bladder cancers associated with schistosomiasis. Jpn J Cancer Res. 1987 Sep;78(9):915–920. [PubMed] [Google Scholar]
- Fujiwara T., Grimm E. A., Mukhopadhyay T., Zhang W. W., Owen-Schaub L. B., Roth J. A. Induction of chemosensitivity in human lung cancer cells in vivo by adenovirus-mediated transfer of the wild-type p53 gene. Cancer Res. 1994 May 1;54(9):2287–2291. [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Hong S. J., Lee T., Park Y. S., Lee K. O., Chung B. H., Lee S. H. A PCR-RFLP method for the detection of activated H-ras oncogene with a point mutation at codon 12 and 61. Yonsei Med J. 1996 Dec;37(6):371–379. doi: 10.3349/ymj.1996.37.6.371. [DOI] [PubMed] [Google Scholar]
- Jaros E., Perry R. H., Adam L., Kelly P. J., Crawford P. J., Kalbag R. M., Mendelow A. D., Sengupta R. P., Pearson A. D. Prognostic implications of p53 protein, epidermal growth factor receptor, and Ki-67 labelling in brain tumours. Br J Cancer. 1992 Aug;66(2):373–385. doi: 10.1038/bjc.1992.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Tomita Y., Bilim V., Takeda M., Takahashi K., Kumanishi T. Abrogation of apoptosis induced by DNA-damaging agents in human bladder-cancer cell lines with p21/WAF1/CIP1 and/or p53 gene alterations. Int J Cancer. 1996 Nov 15;68(4):501–505. doi: 10.1002/(SICI)1097-0215(19961115)68:4<501::AID-IJC16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Kinzler K. W., Bruskin A., Jarosz D., Friedman P., Prives C., Vogelstein B. Identification of p53 as a sequence-specific DNA-binding protein. Science. 1991 Jun 21;252(5013):1708–1711. doi: 10.1126/science.2047879. [DOI] [PubMed] [Google Scholar]
- Kessis T. D., Slebos R. J., Han S. M., Shah K., Bosch X. F., Muñoz N., Hedrick L., Cho K. R. p53 gene mutations and MDM2 amplification are uncommon in primary carcinomas of the uterine cervix. Am J Pathol. 1993 Nov;143(5):1398–1405. [PMC free article] [PubMed] [Google Scholar]
- Kusser W. C., Miao X., Glickman B. W., Friedland J. M., Rothman N., Hemstreet G. P., Mellot J., Swan D. C., Schulte P. A., Hayes R. B. p53 mutations in human bladder cancer. Environ Mol Mutagen. 1994;24(3):156–160. doi: 10.1002/em.2850240303. [DOI] [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Perry M. E., Chang A., Silver A., Dittmer D., Wu M., Welsh D. The 1993 Walter Hubert Lecture: the role of the p53 tumour-suppressor gene in tumorigenesis. Br J Cancer. 1994 Mar;69(3):409–416. doi: 10.1038/bjc.1994.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipponen P. K., Liukkonen T. J. Reduced expression of retinoblastoma (Rb) gene protein is related to cell proliferation and prognosis in transitional-cell bladder cancer. J Cancer Res Clin Oncol. 1995;121(1):44–50. doi: 10.1007/BF01202728. [DOI] [PubMed] [Google Scholar]
- Livingstone L. R., White A., Sprouse J., Livanos E., Jacks T., Tlsty T. D. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992 Sep 18;70(6):923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Mazars G. R., Jeanteur P., Lynch H. T., Lenoir G., Theillet C. Nucleotide sequence polymorphism in a hotspot mutation region of the p53 gene. Oncogene. 1992 Apr;7(4):781–782. [PubMed] [Google Scholar]
- Neal D. E., Sharples L., Smith K., Fennelly J., Hall R. R., Harris A. L. The epidermal growth factor receptor and the prognosis of bladder cancer. Cancer. 1990 Apr 1;65(7):1619–1625. doi: 10.1002/1097-0142(19900401)65:7<1619::aid-cncr2820650728>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Pavletich N. P., Chambers K. A., Pabo C. O. The DNA-binding domain of p53 contains the four conserved regions and the major mutation hot spots. Genes Dev. 1993 Dec;7(12B):2556–2564. doi: 10.1101/gad.7.12b.2556. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues N. R., Rowan A., Smith M. E., Kerr I. B., Bodmer W. F., Gannon J. V., Lane D. P. p53 mutations in colorectal cancer. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7555–7559. doi: 10.1073/pnas.87.19.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky D., Von Eschenbach A., Tsai Y. C., Jones P., Summerhayes I., Marshall F., Paul M., Green P., Hamilton S. R., Frost P. Identification of p53 gene mutations in bladder cancers and urine samples. Science. 1991 May 3;252(5006):706–709. doi: 10.1126/science.2024123. [DOI] [PubMed] [Google Scholar]
- Sjögren S., Inganäs M., Norberg T., Lindgren A., Nordgren H., Holmberg L., Bergh J. The p53 gene in breast cancer: prognostic value of complementary DNA sequencing versus immunohistochemistry. J Natl Cancer Inst. 1996 Feb 21;88(3-4):173–182. doi: 10.1093/jnci/88.3-4.173. [DOI] [PubMed] [Google Scholar]
- Spruck C. H., 3rd, Rideout W. M., 3rd, Olumi A. F., Ohneseit P. F., Yang A. S., Tsai Y. C., Nichols P. W., Horn T., Hermann G. G., Steven K. Distinct pattern of p53 mutations in bladder cancer: relationship to tobacco usage. Cancer Res. 1993 Mar 1;53(5):1162–1166. [PubMed] [Google Scholar]
- Takahashi T., Takahashi T., Suzuki H., Hida T., Sekido Y., Ariyoshi Y., Ueda R. The p53 gene is very frequently mutated in small-cell lung cancer with a distinct nucleotide substitution pattern. Oncogene. 1991 Oct;6(10):1775–1778. [PubMed] [Google Scholar]
- Uchida T., Wada C., Ishida H., Wang C., Egawa S., Yokoyama E., Kameya T., Koshiba K. p53 mutations and prognosis in bladder tumors. J Urol. 1995 Apr;153(4):1097–1104. [PubMed] [Google Scholar]
- Vageli D., Kiaris H., Delakas D., Anezinis P., Cranidis A., Spandidos D. A. Transcriptional activation of H-ras, K-ras and N-ras proto-oncogenes in human bladder tumors. Cancer Lett. 1996 Oct 22;107(2):241–247. doi: 10.1016/0304-3835(96)04372-8. [DOI] [PubMed] [Google Scholar]
- Vet J. A., Bringuier P. P., Poddighe P. J., Karthaus H. F., Debruyne F. M., Schalken J. A. p53 mutations have no additional prognostic value over stage in bladder cancer. Br J Cancer. 1994 Sep;70(3):496–500. doi: 10.1038/bjc.1994.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vet J. A., Bringuier P. P., Schaafsma H. E., Witjes J. A., Debruyne F. M., Schalken J. A. Comparison of P53 protein overexpression with P53 mutation in bladder cancer: clinical and biologic aspects. Lab Invest. 1995 Dec;73(6):837–843. [PubMed] [Google Scholar]
- Zhan Q., Carrier F., Fornace A. J., Jr Induction of cellular p53 activity by DNA-damaging agents and growth arrest. Mol Cell Biol. 1993 Jul;13(7):4242–4250. doi: 10.1128/mcb.13.7.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]