Abstract
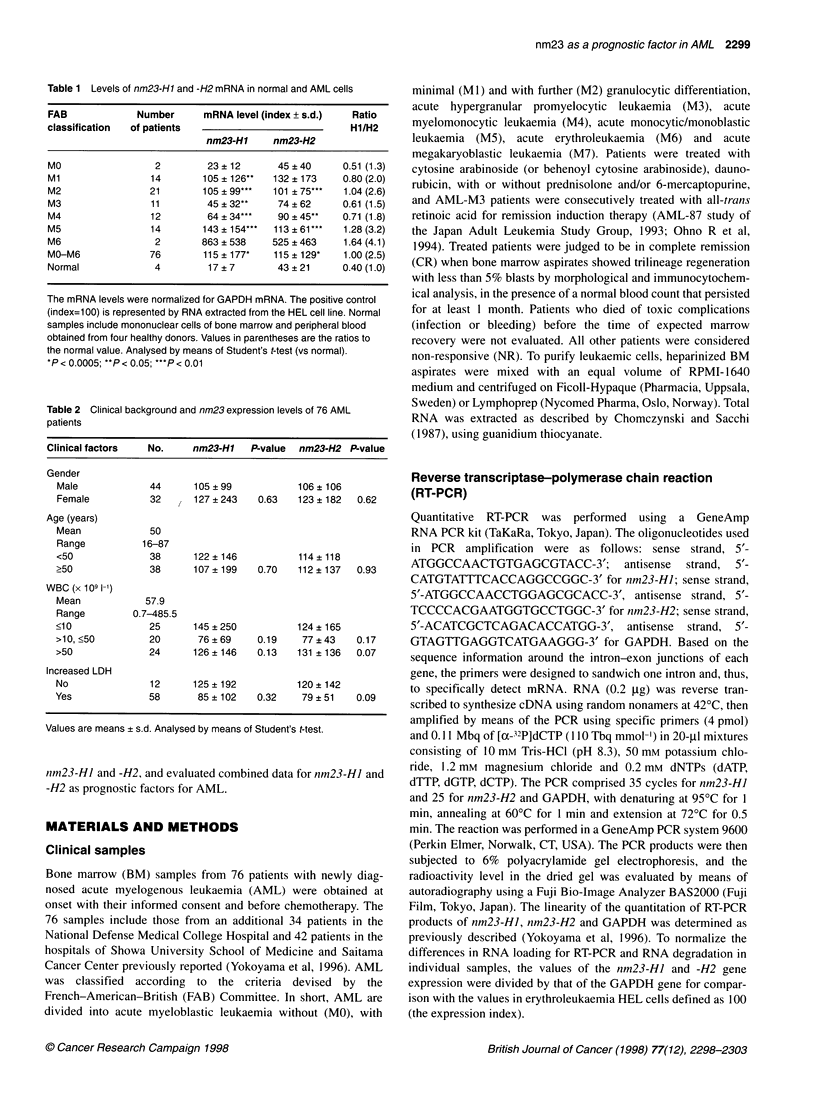
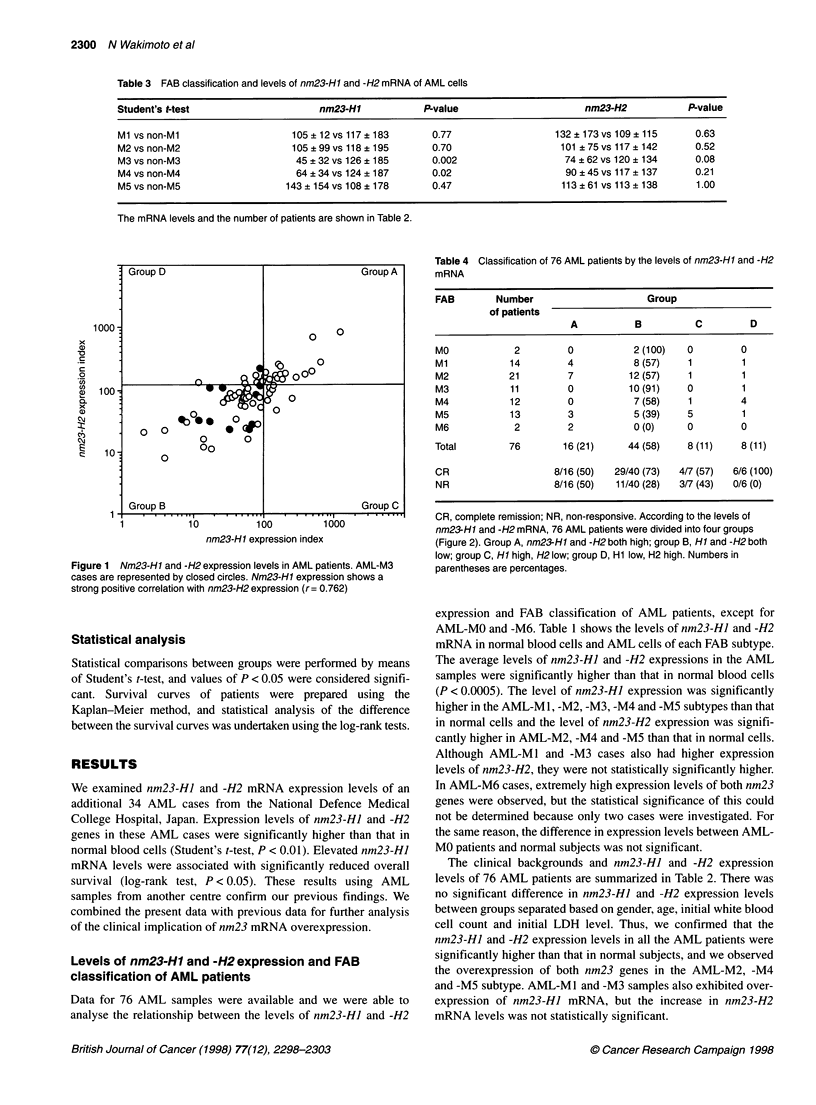
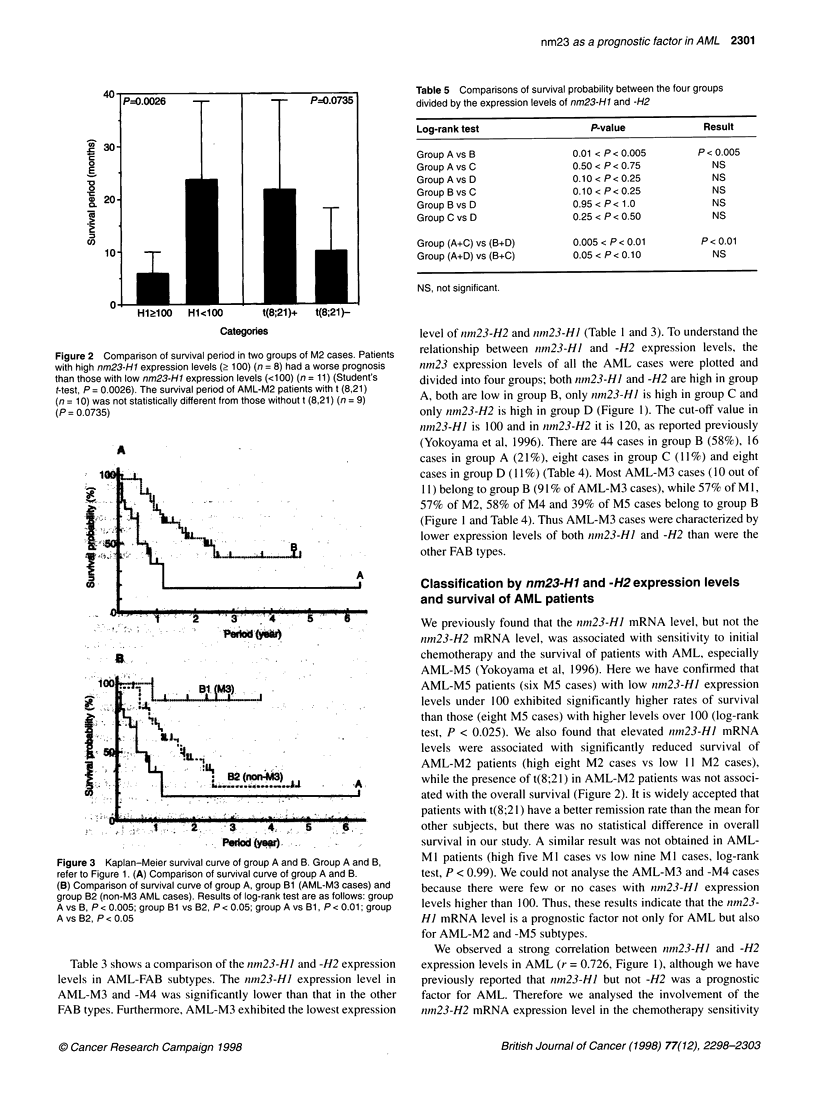
Differentiation inhibitory factor (nm23 protein) inhibited the induction of the differentiation of various leukaemic cell lines. We previously reported that nm23 genes (H1 and H2) were overexpressed in acute myelogenous leukaemia (AML) and nm23-H1 expression predicted the prognosis of AML, especially AML-M5. To clarify the correlation between French-American-British (FAB) classification and nm23 expression level and to clarify the involvement of nm23-H2 and nm23-H1 in patient survival, we investigated the relative levels of nm23-H1 and -H2 mRNA in 76 AML samples using the reverse transcriptase-polymerase chain reaction. We confirmed that the expression of both nm23-H1 and -H2 genes in AML samples from three different hospitals was significantly higher than that in normal blood cells (P < 0.0005). Overexpression of nm23-H1 was observed in each FAB AML-M1, -M2, -M3, -M4 or -M5 subtype, and the predictive effect of nm23-H1 expression on AML prognosis was shown in FAB AML-M2 and -M5 cases. Although overexpression of nm23-H2 was also found in each FAB subtype, the expression of nm23-H2 in AML-M1 and -M3 cells was not significantly higher than that in normal cells. Among AML subtypes, AML-M3 showed the lowest expression levels of both nm23 genes. To understand the relationship between nm23-H1 and -H2 expression levels, nm23 expression levels for all the AML cases were plotted and divided into four groups (group A, nm23-H1 and -H2 both high; B, both low; C, only nm23-H1 high; D, only nm23-H2 high). A statistically significant correlation between the levels of expression of nm23-H1 and -H2 was observed (r= 0.726). Most AML-M3 cases belonged to group B, but not other types of AML. Analysis of survival probability between the groups showed that group B survived for significantly longer compared with group A. Furthermore, AML-M3 cases survived for significantly longer compared with non-M3 cases in the same group B. These data suggest that low expression levels of both nm23-H1 and -H2 are associated with good prognosis in AML patients.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backer J. M., Mendola C. E., Kovesdi I., Fairhurst J. L., O'Hara B., Eddy R. L., Jr, Shows T. B., Mathew S., Murty V. V., Chaganti R. S. Chromosomal localization and nucleoside diphosphate kinase activity of human metastasis-suppressor genes NM23-1 and NM23-2. Oncogene. 1993 Feb;8(2):497–502. [PubMed] [Google Scholar]
- Chandrasekharappa S. C., Gross L. A., King S. E., Collins F. S. The human NME2 gene lies within 18kb of NME1 in chromosome 17. Genes Chromosomes Cancer. 1993 Apr;6(4):245–248. doi: 10.1002/gcc.2870060411. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Gilles A. M., Presecan E., Vonica A., Lascu I. Nucleoside diphosphate kinase from human erythrocytes. Structural characterization of the two polypeptide chains responsible for heterogeneity of the hexameric enzyme. J Biol Chem. 1991 May 15;266(14):8784–8789. [PubMed] [Google Scholar]
- Okabe-Kado J., Kasukabe T., Honma Y., Hayashi M., Henzel W. J., Hozumi M. Identity of a differentiation inhibiting factor for mouse myeloid leukemia cells with NM23/nucleoside diphosphate kinase. Biochem Biophys Res Commun. 1992 Feb 14;182(3):987–994. doi: 10.1016/0006-291x(92)91829-f. [DOI] [PubMed] [Google Scholar]
- Okada K., Urano T., Baba H., Furukawa K., Furukawa K., Shiku H. Independent and differential expression of two isotypes of human Nm23: analysis of the promoter regions of the nm23-H1 and H2 genes. Oncogene. 1996 Nov 7;13(9):1937–1943. [PubMed] [Google Scholar]
- Okada K., Urano T., Goi T., Baba H., Yamaguchi A., Furukawa K., Shiku H. Isolation of human nm23 genomes and analysis of loss of heterozygosity in primary colorectal carcinomas using a specific genomic probe. Cancer Res. 1994 Aug 1;54(15):3979–3982. [PubMed] [Google Scholar]
- Paravicini G., Steinmayr M., André E., Becker-André M. The metastasis suppressor candidate nucleotide diphosphate kinase NM23 specifically interacts with members of the ROR/RZR nuclear orphan receptor subfamily. Biochem Biophys Res Commun. 1996 Oct 3;227(1):82–87. doi: 10.1006/bbrc.1996.1471. [DOI] [PubMed] [Google Scholar]
- Steeg P. S., Bevilacqua G., Kopper L., Thorgeirsson U. P., Talmadge J. E., Liotta L. A., Sobel M. E. Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst. 1988 Apr 6;80(3):200–204. doi: 10.1093/jnci/80.3.200. [DOI] [PubMed] [Google Scholar]
- Yamashiro S., Urano T., Shiku H., Furukawa K. Alteration of nm23 gene expression during the induced differentiation of human leukemia cell lines. Oncogene. 1994 Sep;9(9):2461–2468. [PubMed] [Google Scholar]
- Yokoyama A., Okabe-Kado J., Sakashita A., Maseki N., Kaneko Y., Hino K., Tomoyasu S., Tsuruoka N., Kasukabe T., Honma Y. Differentiation inhibitory factor nm23 as a new prognostic factor in acute monocytic leukemia. Blood. 1996 Nov 1;88(9):3555–3561. [PubMed] [Google Scholar]
- de la Rosa A., Williams R. L., Steeg P. S. Nm23/nucleoside diphosphate kinase: toward a structural and biochemical understanding of its biological functions. Bioessays. 1995 Jan;17(1):53–62. doi: 10.1002/bies.950170111. [DOI] [PubMed] [Google Scholar]


