Abstract
The type 3 ryanodine receptor (RyR3) is a ubiquitous calcium release channel that has recently been found in mammalian skeletal muscles. However, in contrast to the skeletal muscle isoform (RyR1), neither the subcellular distribution nor the physiological role of RyR3 are known. Here, we used isoform-specific antibodies to localize RyR3 in muscles of normal and RyR knockout mice. In normal hind limb and diaphragm muscles of young mice, RyR3 was expressed in all fibers where it was codistributed with RyR1 and with the skeletal muscle dihydropyridine receptor. This distribution pattern indicates that RyR3 is localized in the triadic junctions between the transverse tubules and the sarcoplasmic reticulum. During development, RyR3 expression declined rapidly in some fibers whereas other fibers maintained expression of RyR3 into adulthood. Comparing the distribution of RyR3-containing fibers with that of known fiber types did not show a direct correlation. Targeted deletion of the RyR1 or RyR3 gene resulted in the expected loss of the targeted isoform, but had no adverse effects on the expression and localization of the respective other RyR isoform. The localization of RyR3 in skeletal muscle triads, together with RyR1, is consistent with an accessory function of RyR3 in skeletal muscle excitation–contraction coupling.
Keywords: skeletal muscle, calcium release channel, excitation–contraction coupling, immunofluorescence, knockout mice
Ryanodine receptors (RyR)1 comprise a class of three calcium release channels that are encoded by separate genes (Sorrentino 1995; Sutko and Airey 1996). The skeletal muscle isoform type 1 (RyR1) and the cardiac isoform type 2 (RyR2) are essential for excitation–contraction coupling in skeletal and heart muscle, respectively (Marks et al. 1989; Takeshima et al. 1989; Nakai et al. 1990; Otsu et al. 1990; Zorzato et al. 1990). The type 3 isoform (RyR3) is expressed in many tissues, including the brain and skeletal muscles (Giannini et al. 1992, Giannini et al. 1995). However, the physiological role of RyR3 is not known.
In skeletal muscle RyR1 is localized in the triad junction, where it closely interacts with the dihydropyridine receptor in depolarization-induced calcium release from the sarcoplasmic reticulum (SR; Flucher and Franzini-Armstrong 1996; Franzini-Armstrong and Protasi 1997). In response to depolarization of the transverse (T)-tubules, the dihydropyridine receptor, the physiological voltage-sensor, rapidly activates the opening of the RyR1 without the necessity of conducting calcium by itself (Melzer et al. 1995). In turn, the dihydropyridine receptor receives signals from the RyR1 that modulate its L-type calcium channel properties (Fleig et al. 1996; Nakai et al. 1996). In order for such a close interaction to occur, the two channels are coordinately arranged in the opposing junctional membranes (Block et al. 1988). The clover leaf shaped cytoplasmic foot of every other RyR homotetramer in the SR membrane faces a group of four dihydropyridine receptors, called tetrads, in the T-tubule membrane. Current models of skeletal muscle excitation–contraction coupling regard the dihydropyridine receptor-coupled RyRs as directly under the control of membrane depolarization, and the uncoupled RyRs as indirectly regulated by calcium-induced calcium release (Stern et al. 1997).
Biochemical studies have shown that most of avian, amphibian, and fish skeletal muscles contain two RyR isoforms, α and β (Sutko et al. 1991), which correspond to mammalian RyR1 and RyR3, respectively (Oyamada et al. 1994; Ottini et al. 1996). Recent evidence indicates that RyR3 is also expressed in mammalian muscles, although at lower levels than RyR1 (Giannini et al. 1992, Giannini et al. 1995). In mammals, the two isoforms are differentially expressed during development, as well as in different muscle types (Conti et al. 1996; Bertocchini et al. 1997; Tarroni et al. 1997). RyR3 is detected by Western blot analysis in all skeletal muscles of late embryonic stage and during the first two weeks after birth, however, it is downregulated in most mammalian muscles of adult animals with the exception of diaphragm and soleus muscles.
A central role for the RyR1 isoform in skeletal muscle is indicated by the observation that homozygous RyR1 knockout mice die upon birth from respiratory failure (Takeshima et al. 1994). The phenotype of these mice is similar to that of the cn/cn chicken mutant which also does not express RyR1 (Ivanenko et al. 1995). In both cases, excitation–contraction coupling is lost despite the fact that the muscle cells express considerable amounts of RyR3 (Ivanenko et al. 1995; Takeshima et al. 1995). In agreement with this observation, expression of exogenous RyR2 and RyR3 in RyR1−/− myotubes did not rescue excitation–contraction coupling. Together, these studies demonstrated that only RyR1 is capable of establishing a functional coupling with the voltage sensor. In contrast, all three channel isoforms can be activated by calcium (Yamazawa et al. 1996).
At variance with RyR1 knockout, RyR3 knockout mice are viable and have no major defect of muscle function (Takeshima et al. 1996). However, consistent with the neonatal pattern of RyR3 expression, studies on contractile properties of RyR3 knockout muscles revealed that the amount of force generated upon electrical stimulation or following caffeine exposure was strongly depressed in skeletal muscles from newborn RyR3−/− mice compared with normal controls (Bertocchini et al. 1997). This suggests that, at least during early development, RyR3 plays a role in the amplification of the calcium signal in excitation–contraction coupling.
To understand the role of RyR3 in skeletal muscle function, it is important to answer the question as to whether RyR3 is localized in the triad junction or elsewhere in the muscle fiber, i.e., in extrajunctional calcium stores. Further, it is important to determine whether all fibers express both RyR1 and RyR3 simultaneously during early development and then downregulate RyR3 to different degrees, or whether RyR3 is expressed exclusively by a subset of fibers, which are gradually replaced by RyR1-containing fibers. To this end, we conducted an immunolocalization study comparing the expression and subcellular distribution of RyR1 and RyR3 at different developmental stages in muscles of normal and RyR knockout mice. The results of this study indicate that RyR3 is localized in the triad junction and coexpressed with RyR1 in the same muscle fibers, indicative of an accessory role of RyR3 in skeletal muscle excitation–contraction coupling.
Materials and Methods
Mice Strains
Generation of RyR1−/− and RyR3−/− mice has been described elsewhere (Takeshima et al. 1994; Bertocchini et al. 1997). For tissue preparation, normal, homozygous RyR1−/−, and homozygous RyR3−/− mice were anesthetized with carbon dioxide, decapitated, and tissue samples were removed. Small muscle pieces were infiltrated in 5% DMSO in PBS for 5 to 15 min and then rapidly frozen in precooled 2-methylbutane and stored at −70°C. Frozen samples were mounted in OCT compound, sectioned on a cryostat, and the sections were mounted on glass microscope slides.
Immunofluorescence Labeling
Tissue sections were rinsed in PBS containing 0.2% BSA and 0.2% Triton X-100 (PBS/BSA/Triton) and then incubated in 5% normal goat serum (NGS) in PBS/BSA/Triton for 30 min at room temperature. Then, the sections were incubated in primary antibody for 4 h at room temperature or overnight at 4°C and subsequently washed with five changes of PBS/BSA/Triton for a total of 30 min at room temperature. Sections were then incubated in fluorochrome-conjugated secondary antibody for 1 h at room temperature and washed as before. The sections were then incubated in 90% glycerol, 0.1 M Tris, pH 8, with 5 mg/ml p-phenylenediamine to retard photobleaching and covered with a coverglass.
The following primary antibodies were used at the indicated concentrations: affinity-purified rabbit antibody against RyR1 at 1:5,000 (AB#5; Flucher et al. 1993); rabbit antiserum against RyR3 at 1:1,000; monoclonal mouse antibody against the skeletal muscle dihydropyridine receptor α1 subunit at 0.1 mM (mAb 1A; Morton and Froehner 1987); monoclonal mouse antibody against fast isoform of the skeletal muscle Ca2+ ATPase, SERCA1 at 1:500 (MA3-911; Affinity BioReagents; Jorgensen et al. 1988); and monoclonal mouse antibodies against the following isoforms of myosin heavy chain, which were generously supplied by Dr. S. Schiaffino (University of Padova, Italy): antibody BF-G6 against embryonic MHC used at 1:4,000; BA-D5 against slow MHC used at 1:500; and SC-71 against fast red, 2A MHC used at 1:500. Secondary antibodies were Cy3- and fluorescein-conjugated goat anti–rabbit and goat anti–mouse IgG (Jackson ImmunoResearch Laboratories) used at a dilution of 1:5,000. All antibodies have been characterized elsewhere and, except anti-RyR3, extensively used in immunocytochemistry (Flucher et al. 1993, Flucher et al. 1994; Powell et al. 1996).
As controls, primary antibodies were omitted or inappropriate secondary antibodies were applied. Isoform-specificity of the RyR antibodies was evaluated in situ using tissues from the homozygous RyR1−/−, RyR3−/− (see Results), and from a double knockout strain of mice for both RyR1 and RyR3 genes (RyR1−/− and RyR3−/−; not shown; Barone et al. 1998). Immunostained sections were evaluated and analyzed on a Zeiss fluorescence microscope and images were captured with a Zeiss laser scanning confocal microscope using the 63×, 1.4 NA, Plan-Apochromat objective lens.
Microsomal Vesicle Preparations and Western Blot Analysis
Skeletal muscles isolated from mice at indicated ages were used to prepare the microsomal fractions as previously described (Conti et al. 1996). Muscles were homogenized in ice-cold buffer A (320 mM sucrose, 5 mM Na-Hepes, pH 7.4, and 0.1 mM PMSF) using a Dounce homogenizer. Homogenates were centrifuged at 7,000 g for 5 s at 4°C. The supernatant obtained was centrifuged at 100,000 g for 1 h at 4°C. The microsomes were resuspended in buffer A and stored at −80°C. Protein concentration of the microsomal fraction was quantified using the Bradford protein assay kit (BioRad). Microsomal proteins were separated by SDS/PAGE and then transferred to a nitrocellulose membrane (Schleicher & Schuell). Membranes were incubated for 3 h in 150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 0.2% Tween 20, plus 5% nonfat milk. Primary antibodies used were polyclonal rabbit antisera (diluted 1:3,000) against the RyR isoform (Giannini et al. 1995). Antigen detection was performed using the amplified alkaline phosphatase detection method.
Results
Expression of RyR1 and RyR3 in Embryonic Muscles of Wild-type and Knockout Mice
To determine to what extent RyR3 is expressed in developing skeletal muscle and whether it is coexpressed with RyR1 in the same fibers, unfixed cryosections of muscles from normal, homozygous RyR1−/−, and homozygous RyR3−/− mice at embryonic day 18 (E18) were immunolabeled with specific antibodies against RyR1 and RyR3 (Fig. 1). In wild-type muscles, both RyR antibodies stained all myofibers with similar intensity, indicating that, at this developmental stage, RyR1 and RyR3 are coexpressed in mouse skeletal muscles. The labeling patterns for both RyR isoforms were punctate and irregularly distributed throughout the myoplasm (Fig. 1, a and b), resembling the distribution pattern of triad proteins that is typically found in E18 muscle fibers. Since the nuclei were still centrally located in the myofibers, the labeling pattern appeared ring-shaped in cross-sections. RyR1−/− muscles were labeled with anti-RyR3, but not with anti-RyR1 (Fig. 1c and Fig. d). Conversely, RyR3−/− muscles were labeled with anti-RyR1, but not with anti-RyR3 (Fig. 1e and Fig. f). This is consistent with previous immunoblot experiments (Bertocchini et al. 1997) and shows that there are no cross-reactions of anti-RyR1 with RyR3 and of anti-RyR3 with RyR1. Thus, the immunofluorescence assay is highly specific for the respective RyR isoforms. Furthermore, the absence of immunostain with anti-RyR3 and anti-RyR1 in muscles of RyR1−/− and RyR3−/− mice, respectively, provides additional evidence that the targeted mutations of the genes encoding the RyR isoforms resulted in the complete and specific loss of the respective proteins. Expression of RyR3 in skeletal muscles of RyR1−/− mice was also observed in a second independent RyR1 knockout mouse strain (data not shown) generated by Dr. P.D. Allen (Brigham and Women's Hospital, Boston, MA). Normal expression of RyR1 in RyR3−/− mice was also detected in skeletal muscles from mice 15-, 25-, and 60-d-old (D15, D25, and D60; not shown).
Figure 1.
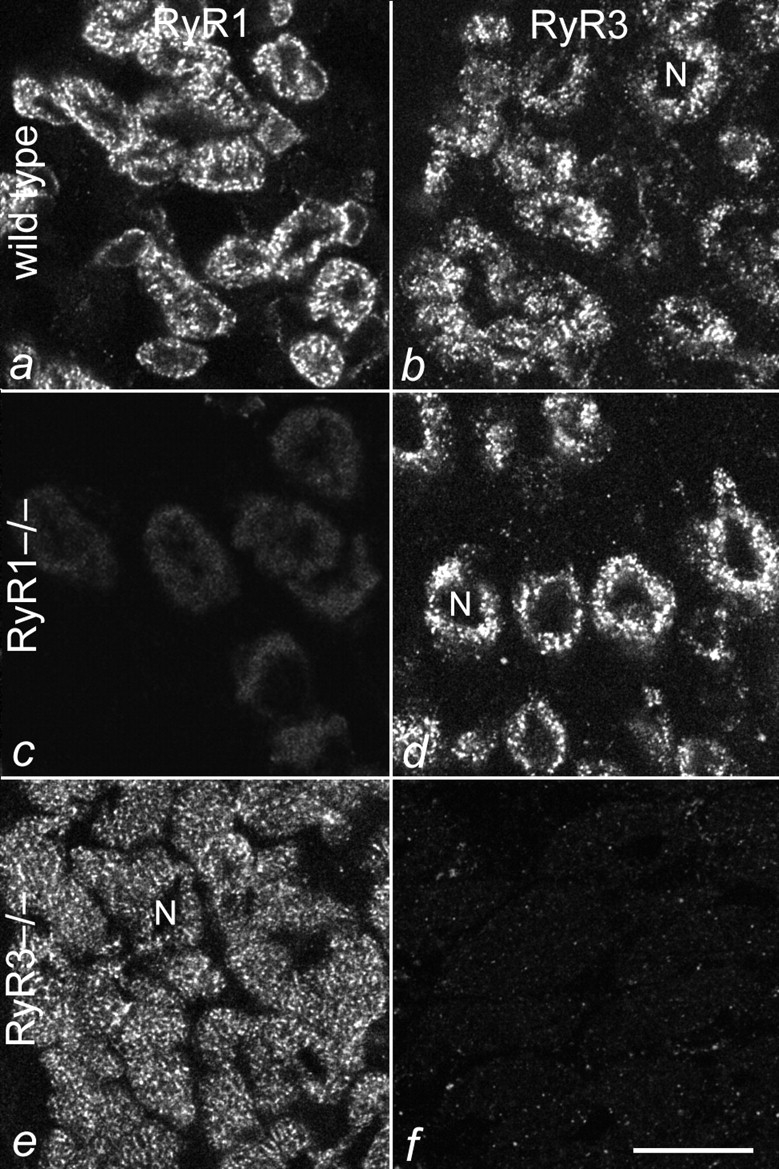
Expression of RyR1 and RyR3 in E18 muscles of wild-type and RyR knockout mice. Cross-sections of normal (a and b), RyR1−/− (c and d), and RyR3−/− (e and f) hind limb muscles were immunofluorescence-labeled with specific antibodies against RyR1 (a, c, and e) and RyR3 (b, d, and f). RyR1 is expressed in all fibers of wild-type and RyR3−/− muscles, but not in RyR1−/− muscles. RyR3 is expressed in all fibers of wild-type and RyR1−/− muscles, but not in RyR3−/− muscles. No cross-reaction between anti-RyR1 and RyR3, or anti-RyR3 and RyR1 was observed. N, centrally located nuclei. Bar, 20 mm.
RyR3 and RyR1 Are Localized in Triads
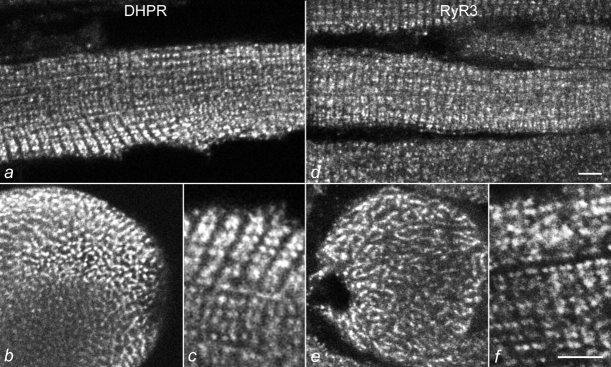
If RyR3 is involved in the initial aspects of excitation–contraction coupling, then its localization in the triad would be expected. With fluorescent microscopy, triad proteins exhibit characteristic labeling patterns (Fig. 2 and Fig. 3). In longitudinal sections of differentiated skeletal muscles, a cross-striated banding pattern with a center-to-center distance between the bands of ∼2 μm can be seen. At higher resolution, the cross-striated bands can be identified as double rows of fluorescent dots, representing the pairs of triads on either sides of the Z-line. Pairs of neighboring fluorescent dots are aligned along the myofibril bundles. In cross-sections, triad proteins show a network of membranes encircling the myofibril bundles. Fig. 2 compares the distribution pattern of a known triad protein, the α1 subunit of the dihydropyridine receptor (Fig. 2, a–c), with that of RyR3 (Fig. 2, d–f) in D15 normal diaphragm muscle. The distribution of the RyR3 immunolabel shows the characteristics of a triad protein, most importantly the double rows of fluorescent dots (Fig. 2 f). The fact that a T-tubule protein, the dihydropyridine receptor, and a SR protein, RyR3 (or RyR1) stain the same cytoplasmic structure is further evidence that this structure represents the T-tubule/SR junction, i.e., the triad. No other cytoplasmic structure showed significant RyR3 label. Thus, RyR3 is localized in the triad junctions of skeletal muscle and not to any significant amount in other regions of the ER/SR system.
Figure 2.
Colocalization of RyR3 and the skeletal muscle dihydropyridine receptor in mouse skeletal muscle. Cryostat sections of D15 normal mouse diaphragm muscles were immunolabeled with specific antibodies against the α1 subunit of the dihydropyridine receptor (a–c) and against RyR3 (d–f). Longitudinal sections (a, c, d, and f) show cross-striations of double rows of fluorescent dots with both antibodies. Cross-sections (b and e) reveal a labeled meshwork of tubular structures. The colocalization of a junctional T-tubule protein (dihydropyridine receptor) with the RyR3 identifies the labeled structure as T-tubule/SR junction. Bars, 5 mm.
Figure 3.
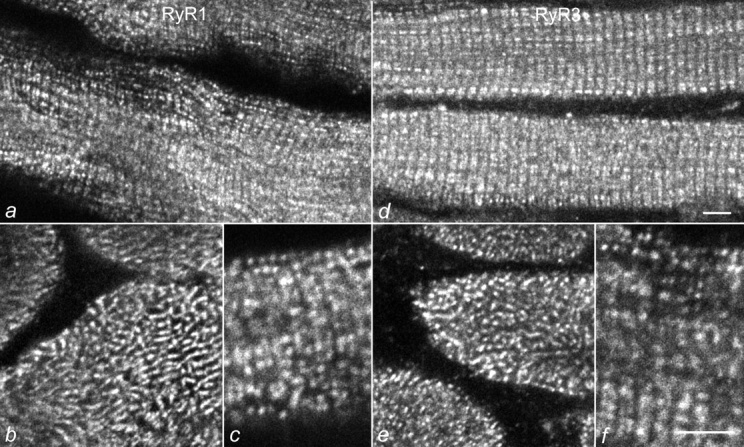
Colocalization of RyR1 and RyR3 in young mouse skeletal muscle. Sections of D15 normal mouse diaphragm muscles were immunolabeled with specific antibodies against RyR1 (a–c) and RyR3 (d–f). Both antibodies show the typical labeling pattern of triad proteins: double rows of fluorescent dots representing the pairs of triads on either sides of the Z-line or the meshwork between the myofibrillar bundles, in longitudinally (a, c, d, and f) and cross-sectioned (b and e) muscle fibers, respectively. Bars, 5 mm.
The coextensive labeling patterns of RyR1 and RyR3 observed in E18 hind limb muscles (Fig. 1) combined with the localization of RyR3 immunolabel in the triads of differentiated muscle (Fig. 2) suggests that the two RyR isoforms coexist in the same triads. Fig. 3 allows the direct comparison of the subcellular distribution of RyR3 with that of RyR1 in D15 normal mouse diaphragm. Specific antibodies against RyR1 and RyR3 labeled all muscle fibers to the same extent and the two RyR isoforms showed identical triad labeling patterns in longitudinal and cross-sections. Thus, RyR3 and RyR1 coexist in, or in close proximity to, young skeletal muscle triads. Triad staining of RyR3, as shown for D15 diaphragm in Fig. 3 was also observed in RyR3-expressing fibers from hind limb muscles (not shown) and from muscles of later developmental stages. However, in older muscles, RyR3 was not expressed uniformly in all muscle fibers, but only in a subset of fibers. Fig. 4 shows an example of normal D25 diaphragm labeled with antibodies against RyR1 and RyR3. Whereas anti-RyR1 uniformly stained all muscle fibers in the fields, only one fiber in each field is stained with anti-RyR3. These RyR3-positive muscle fibers were labeled as intensively as those of D15 and also expressed RyR1 (Fig. 4c and Fig. d). This shows that, during postnatal development, the majority of muscle fibers lose RyR3 from their triads. However, those fibers that continue to express RyR3 also express RyR1.
Figure 4.
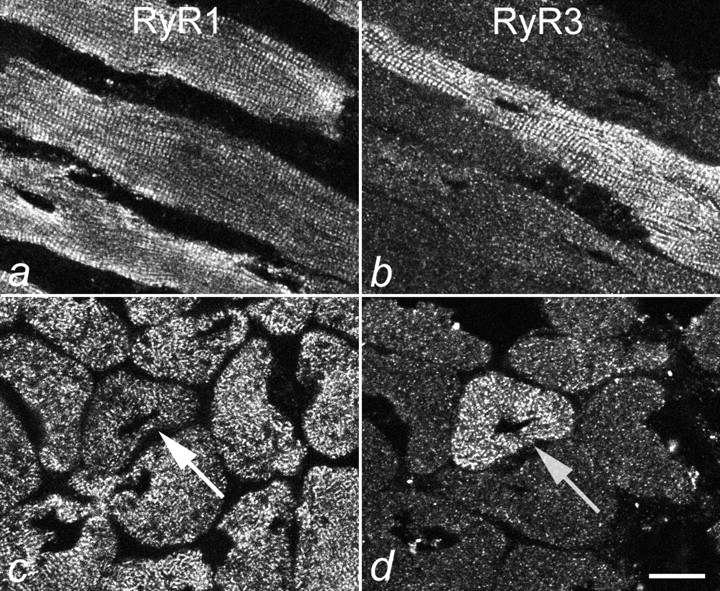
Differential expression of RyR1 and RyR3 in D25 diaphragm muscle. Longitudinal and cross-sections of D25 mouse diaphragm muscle were immunolabeled with antibodies against RyR1 (a and c) and RyR3 (b and d). Anti-RyR1 stains all muscle fibers, but anti-RyR3 stains only a fraction of the muscle fibers at this developmental stage. Serial sections (c and d) show that the fiber that expresses RyR3 (d) also contains RyR1 (c). Bar, 10 mm.
Differential Downregulation of RyR3 in Diaphragm and Hind Limb Muscles
The finding described above indicates that the RyR3 content does not decrease gradually and simultaneously in all muscle fibers, but that it decreases rapidly in some fibers and not in others. Fig. 5 shows that during postnatal development the number of RyR3-containing fibers dramatically decreases with age and that this occurs at different rates in different muscles. Whereas RyR3-positive fibers could be found even in adult (D60) diaphragm muscle, extensor digitorum longus (EDL) muscle was devoid of RyR3-positive fibers as early as D25. Semiquantitative analysis showed that in diaphragm, the decline in numbers of RyR3-containing fibers did not begin until after D15. By D60, only 13% of the fibers were positive for RyR3. In contrast, in EDL the fraction of RyR3-containing fibers declined to 17% by D15 and disappeared before D25. The overall morphology of the RyR3-containing fibers gave no indication that these fibers were different or at a different stage of differentiation than the neighboring RyR3-lacking fibers in the section. Comparison of the tissue distribution of RyR3 with the fiber type composition of the studied muscles did not suggest a correlation of the RyR3-containing fibers with a distinct fiber type (Conti et al. 1996). Comparison of the fractions of RyR3-containing fibers with fibers labeled with specific antibodies against myosin heavy chain isoforms of embryonic, slow, and fast (2A) fibers, or with an antibody against the fast calcium ATPase, also failed to show any correlation of RyR3 expression with a specific fiber type (Table ). For instance, in D15 diaphragm, 100% of the fibers were positive for RyR3, but only 10%–80% of the fibers reacted with any one of the fiber type markers. Conversely, in D25 EDL muscle, none of the fibers contained RyR3, whereas between 5 and 30% of the fibers reacted with one of the tested markers. As to the onset of RyR expression during embryonic development, we observed both RyR1 and RyR3 in leg muscles fibers at E14 (not shown).
Figure 5.
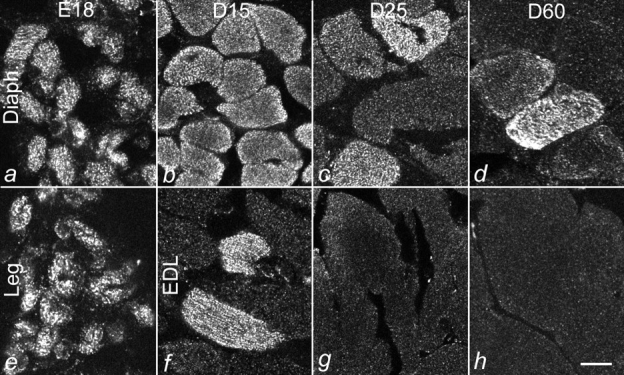
Differential decline of RyR3-expressing muscle fibers in developing diaphragm and hind limb muscles. Cross-sections of diaphragm (a–d), hind limb (e), and extensor digitorum longus (EDL; f–h) muscles from mice at E18 and D15, D25, and D60 were immunolabeled with an antibody against RyR3. In diaphragm, RyR3 was expressed in all fibers up to D15 and was still found in a small fraction of fibers at D60. While E18 hind limb muscle expressed RyR3 in all fibers, RyR3 expression in EDL declined rapidly after birth. Bar, 10 mm.
Table 1.
Comparison of RyR3 Expression and Expression of Fiber Type-specific Myosin Heavy Chains and SR Ca2+ ATPase in Developing Hind Limb and Diaphragm Muscles
| Antibody to | E18 | D15 | D25 | D60 | ||||
|---|---|---|---|---|---|---|---|---|
| Diaphragm | Hind limb | Diaphragm | EDL | Diaphragm | EDL | Diaphragm | EDL | |
| % | % | % | % | % | % | % | % | |
| RyR3 | 100 | 100 | 100 | 16.8 | 21.8 | 0 | 13.4 | 0 |
| Slow MHC | 10.8 | 17.2 | 8.8 | 4.8 | ||||
| Fast MHC (2A fibers) | 38.4 | 31.8 | 41.3 | 27.9 | ||||
| SERCA1 (fast) | 79.9 | 93.2 | ||||||
Number of fibers counted for each condition ranged between 101 and 397.
Western Blot Analysis of RyR1 and RyR3 in Newborn and Adult Diaphragm and Hind Limb Muscles
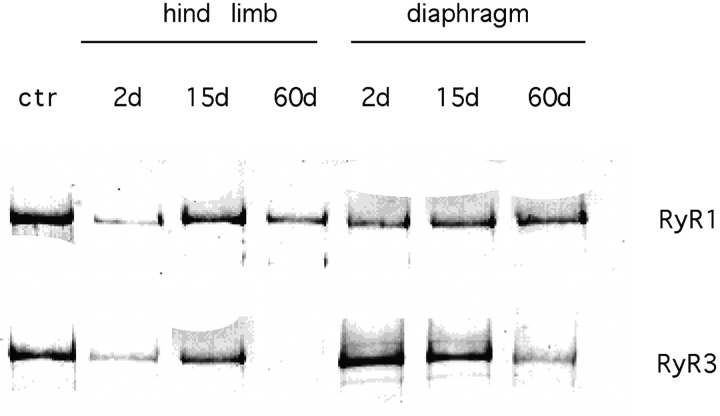
To directly correlate the immunocytochemical data with the relative content of RyR1 and RyR3 in crude membrane preparations of developing hind limb muscles and diaphragm, muscle tissue was isolated from mice at D2 and D15, and from adult mice (i.e., D60) and subsequently analyzed in Western blots. As shown in Fig. 6, the expression patterns of RyR1 and RyR3 differ between hind limb and diaphragm muscles. In neonatal mice, levels of RyR1 and RyR3 are higher in diaphragm than in hind limb muscles. In D2 and D15 diaphragm, RyR3 was found at similar levels, while in hind limb muscles, RyR3 levels increased markedly during the same period. A similar expression pattern (steady expression within the first two weeks after birth in diaphragm and an increase in expression in hind limb muscles) was observed for the RyR1 isoform. In line with previous results, RyR3 expression was reduced below the level of detection in hind limb muscles of adult animals. In contrast, in diaphragm, RyR3 was still detected in adult animals, although at lower levels. These experiments were repeated with five different microsome preparations, always confirming the expression pattern reported in Fig. 6. Densitometric analysis of seven representative Western blots from these experiments indicate a 5–10-fold difference in RyR3 content of diaphragm of newborn and adult mice, which is consistent with the decreased number of muscle fibers expressing RyR3, as observed with immunocytochemistry.
Figure 6.
Developmental expression of the RyR1 and RyR3 isoforms in murine diaphragm and hind limb muscles. Microsomes were prepared from hind limb and diaphragm muscles of 2-d-old (2d), 15-d-old (15d), and adult mice (60d). 3 and 10 μg of microsomal vesicles were loaded for RyR1 and RyR3 immunodetection, respectively. In all experiments, 10 and 30 μg from bovine diaphragm were loaded as control (ctr) for RyR1 and RyR3 immunodetection, respectively. Western blot analysis was performed, as described in the text.
Discussion
Most of our current knowledge of the physiological role of RyRs is derived from studies of RyR1 and RyR2 in striated muscles, where these two RyR isoforms function in depolarization-induced calcium release from the SR. The skeletal muscle RyR1 is controlled directly by the voltage sensor, the dihydropyridine receptor, whereas the cardiac RyR2 opens in response to calcium entering through the dihydropyridine receptor and calcium being released elsewhere from the SR. In contrast, we know little about the biological functions of the ubiquitously expressed RyR3 isoform. Studying RyR3 in skeletal muscle, where we understand the mechanisms of calcium regulation best, may give us important insights into its physiological role.
RyR3 is not essential for muscle function and it cannot substitute for the function of RyR1 in skeletal muscle. Expression of RyR3 is downregulated during early development and some adult mammalian muscles, like EDL, do not express RyR3. Furthermore, RyR3−/− mice develop and move normally. However, depolarization-induced calcium release is weaker in young RyR3−/− muscle fibers than in normal fibers (Bertocchini et al. 1997). Thus, it has been suggested that RyR3 may play an accessory function in skeletal muscle excitation–contraction coupling. This hypothesis is strongly supported by our present finding that RyR3 is localized in skeletal muscle triads. The immunolocalization pattern of RyR3 resembled exactly that of RyR1 in young mammalian muscle fibers and the colocalization of RyR3 with the skeletal muscle dihydropyridine receptor indicates that the RyR3-containing structure corresponds to T-tubule/SR junctions. This finding is in agreement with the preferential distribution of RyR3 in the heavy SR fraction (Conti et al. 1996). Within the triad, the observed immunofluorescence labeling pattern is consistent with one of two possible subcellular localizations. Either RyR3 is interspersed between RyR1 in the junctional face of the terminal SR cisternae, or RyR3 and RyR1 are localized adjacent to each other in separate membrane domains. The alternating organization of the RyRs, with respect to the dihydropyridine receptors (Block et al. 1988), would allow RyR3 to occupy the positions of the uncoupled RyRs within the junction. The preferential association of dihydropyridine receptors with every other RyR foot structure during early formation of junction (Protasi et al. 1997) is suggestive of an alternating organization of two different RyR isoforms within these junctions: one that associates with dihydropyridine receptors and one that does not. However, even if an alternating organization of RyR1 and RyR3 would exist in developing muscle fibers, this can hardly be the mechanism by which this molecular arrangement of the dihydropyridine receptor tetrads is formed, because muscles that do not express RyR3 have been shown to contain alternating coupled and uncoupled RyRs (O'Brien et al. 1993). Alternatively, the RyR3 could occupy regions adjacent to the RyR1-containing junctional domain. This arrangement would be more consistent with the observation that junctions of the RyR1−/− mouse, in which we detect RyR3 with immunofluorescence labeling, apparently lack feet and are significantly narrower than normal T-tubule/SR junctions (Takekura et al. 1995). However, in that and another report (Takeshima et al. 1995), junctions with few feet have occasionally been found. Whether RyR3 is located in one or the other of these two domains of the terminal SR cisternae has implications on current models of excitation–contraction coupling (Stern et al. 1997). But either location, within or adjacent to the junctional face, would be consistent with a role of RyR3 in the amplification of calcium release immediately after initiation of depolarization-induced calcium release.
The fact that RyR3 was not localized in any compartments other than the triad is also important. Even though immunolabeling does not exclude the possibility that RyR3 is expressed at low concentrations in other regions of the SR, its distribution pattern was clearly that of a triad protein and distinct from that of the calcium ATPase, which is concentrated in the longitudinal SR (Jorgensen et al. 1988; Flucher et al. 1993). Thus, it is rather unlikely that RyR3 would primarily serve general housekeeping functions in developing muscles and that its contribution to excitation–contraction coupling would be only secondary. In cardiac ventricular muscle, SR calcium stores have been observed (extended or corbular SR) that contain RyRs, but are not coupled to dihydropyridine receptors (Jorgensen et al. 1993; Carl et al. 1995). Presumably, these calcium release sites serve in the wave-like propagation of the calcium signal into the interior of the cardiac myocytes. The properties of RyR3 would be consistent with a similar function in skeletal muscle, but such structures have not been described in skeletal muscle, and our immunolocalization does not indicate the existence of calcium release sites outside the triads either. Thus, the lack of RyR3 immunolocalization outside the triad junctions further supports the notion that the primary biological role of RyR3 in skeletal muscle is in excitation–contraction coupling. This is in agreement with the results of several recent functional studies of the RyR3 (Chen et al. 1997; Murayama and Ogawa 1997; Sonnleitner et al. 1998), which indicate that RyR3 channels are less sensitive to inactivation at high calcium concentrations than RyR1. Thus, the function of RyR3 in the skeletal muscle triad could be to amplify the calcium signal coming from the directly voltage-activated RyR1 channels (Sorrentino and Reggiani 1999).
The developmental expression of RyR3 differs from that of RyR1. The earliest stage in which we looked for RyR3 expression in skeletal muscle was E14. Even at this time in development, RyR1 and RyR3 were both coexpressed in mouse muscle fibers. This differs from the onset of expression of the corresponding RyR isoform in chicken, where the α RyR precedes the β RyR by as much as five days (Sutko et al. 1991). At E18, all hind limb and diaphragm muscle fibers expressed both RyR isoforms. At this developmental stage, hind limb and diaphragm muscles of RyR1−/− mice also contained normal concentrations of RyR3 (Bertocchini et al. 1997). This finding is consistent with data showing the expression of RyR3 message and the ryanodine and caffeine sensitivity in RyR1−/− myotubes (Takeshima et al. 1995), but it contradicts the results of Buck et al. 1997, who did not detect ryanodine binding or RyR3 immunoreactivity in muscles of a different RyR1 knockout mouse. To rule out that our positive RyR3 immunoreaction resulted from a peculiarity of one particular RyR1 knockout mouse, we performed the immunofluorescence experiments on muscles from RyR1−/− mice generated in two different laboratories (Takeshima et al. 1994; Nakai et al. 1996). In both cases we found RyR3 label in triads of embryonic muscle.
Soon after birth, expression levels of RyR3 began to decline, first in EDL muscle and later in diaphragm. This time course was expected from our earlier results of a Western blot analysis (Bertocchini et al. 1997; Tarroni et al. 1997). However, to our surprise, the loss of the RyR3 isoform in developing muscles occurred not by a continuous decline of RyR3 throughout the muscle, but occurred rapidly in some fibers and delayed or not at all in others.
Available estimates of RyR3 content in adult diaphragm indicate that the content of this isoform varies between 0.7 and 5% of total RyRs in whole muscle homogenates (Murayama and Ogawa 1997; Jeyaknmar et al. 1998). Our Western blot analysis revealed a 5–10-fold reduction in the RyR3 levels of adult diaphragm compared with newborn diaphragm. Immunostaining results presented in Fig. 4 and Fig. 5 show that all fibers of skeletal muscles of fetal and newborn mice contain RyR3, while in adult diaphragm, only a subset of ∼10–15% of the fibers contain RyR3. Together, these findings suggest that the developmental decline in RyR3 protein in adult diaphragm results from the decreased number of RyR3 positive fibers. At the same time, it can be inferred that the content of RyR3 in the subset of fibers expressing this isoform in adult mouse diaphragm is roughly the same as that of newborn fibers. This is of particular importance since it indicates that the relative amount of RyR3 in skeletal muscle fibers that express this isoform is higher than previously appreciated from biochemical estimates based solely on total muscle homogenates.
With the aim of correlating the RyR3-expressing fibers with a functional muscle fiber type, we compared the expression pattern of RyR3 in D15 and D25 diaphragm and EDL muscles with those of different isoforms of the myosin heavy chain and with that of the fast calcium ATPase. However, the expression pattern of none of these marker proteins mirrored that of RyR3, indicating that the regulation of RyR3 expression in developing mammalian muscles is independent of regulation of the fiber-type specific set of proteins. This result is supported by data from an earlier denervation experiment in which no changes of RyR3 expression levels were observed after denervation (Tarroni et al. 1997), even though the fiber type composition is known to change after denervation (Schiaffino et al. 1988). Thus, neither the physiological function of RyR3 nor the regulation of its expression are directly correlated with a single specific fiber type.
The differential regulation of RyR3 expression in different muscle fibers suggests a functional difference between the fibers that contain RyR3 in addition to RyR1 and those that do not. The physiological significance of a second RyR isoform in a skeletal muscle triad is not clear, although expression of two isoforms of RyRs is common in nonmammalian vertebrate skeletal muscles (Sutko and Airey 1996). Two components of Ca2+ release can be identified in voltage clamp experiments: one that is strictly related to membrane depolarization and a second with fast activation and inactivation properties, presumably representing Ca2+-induced Ca2+ release (Rios et al. 1992). While RyR1 alone seems to be able to support both components of Ca2+ release, as they have also been observed in muscle fibers containing only a single RyR isoform, a second RyR isoform with different properties of Ca2+-dependent activation and inactivation may affect the Ca2+ release component that is not under the direct control of membrane depolarization (see Stern et al. 1997).
In summary, the results of this study demonstrate that considerable amounts of RyR3 are expressed in the triads of skeletal muscle where it can perform an accessory function to that of RyR1 in excitation–contraction coupling. Based on the expression pattern in developing muscles, the function of RyR3 appears to be important for all muscles during early development, but only to a subset of muscle fibers of certain adult muscles.
Acknowledgments
We thank Dr. J. Hoflacher for excellent technical assistance, Dr. S. Schiaffino for antibodies, and Dr. C. Reggiani for valuable discussion of the results.
This study was supported by a grant from the Austrian Fonds zur Förderung der Wissenschaftlichen Forschung, Nr. P-12653-MED and by a European Commission's Training and Mobility of Researchers network grant (ERBFMRX-CT96-0032) to B.E. Flucher and by grants from EC (project PL960592), Telethon (grant n.1151), and Agenzia Spaziale Italiana to V. Sorrentino.
Footnotes
1.used in this paper: D, days after birth; E, embryonic day; RyR, ryanodine receptor; SR, sarcoplasmic reticulum; T-tubule, transverse tubule
References
- Barone V., Bertocchini F., Bottinelli R., Protasi F., Allen P.D., Franzini-Armstrong C., Reggiani C., Sorrentino V. Contractile impairment and structural alterations of skeletal muscles from knockout mice lacking type 1 and type 3 ryanodine receptors. FEBS Lett. 1998;422:160–164. doi: 10.1016/s0014-5793(98)00003-9. [DOI] [PubMed] [Google Scholar]
- Bertocchini F., Ovitt C.E., Conti A., Barone V., Schoeler H.R., Bottinelli R., Reggiani C., Sorrentino V. Requirement for the ryanodine receptor type 3 for efficient contraction in neonatal skeletal muscles. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:6956–6963. doi: 10.1093/emboj/16.23.6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block B.A., Imagawa T., Kampbell K.P., Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J. Cell Biol. 1988;107:2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck E.D., Nguyen H.T., Pessah I.N., Allen P.D. Dyspedic mouse skeletal muscle expresses major elements of the triadic junction but lacks detectable ryanodine receptor protein and function. J. Biol. Chem. 1997;272:7360–7367. doi: 10.1074/jbc.272.11.7360. [DOI] [PubMed] [Google Scholar]
- Carl S.L., Felix K., Caswell A.H., Brandt N.R., Ball W.J., Jr., Vaghy P.L., Meissner G., Ferguson D.G. Immunolocalization of sarcolemmal dihydropyridine receptor and sarcoplasmic reticular triadin and ryanodine receptor in rabbit ventricle and atrium. J. Cell Biol. 1995;129:672–682. doi: 10.1083/jcb.129.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.S.R., Li X., Ebisawa K., Zhang L. Functional characterization of the recombinant type 3 Ca2+ release channel (ryanodine receptor) expressed in HEK293 cells. J. Biol. Chem. 1997;272:24234–24246. doi: 10.1074/jbc.272.39.24234. [DOI] [PubMed] [Google Scholar]
- Conti A., Gorza L., Sorrentino V. Differential distribution of ryanodine receptor type 3 (RyR3) gene product in mammalian skeletal muscles. Biochem. J. 1996;316:19–23. doi: 10.1042/bj3160019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleig A., Takeshima H., Penner R. Absence of Ca2+ current facilitation in skeletal muscle of transgenic mice lacking the type 1 ryanodine receptor. J. Physiol. 1996;496:339–345. doi: 10.1113/jphysiol.1996.sp021689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flucher B.E., Franzini-Armstrong C. Formation of junctions involved in excitation–contraction coupling in skeletal and cardiac muscle. Proc. Natl. Acad. Sci. USA. 1996;93:8101–8106. doi: 10.1073/pnas.93.15.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flucher B.E., Andrews S.B., Fleischer S., Marks A.R., Caswell A.H., Powell J.A. Triad formationorganization and function of the sarcoplasmic reticulum calcium release channel and triadin in normal and dysgenic muscle in vitro. J. Cell Biol. 1993;123:1161–1174. doi: 10.1083/jcb.123.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flucher B.E., Andrews S.B., Daniels M.P. Molecular organization of transverse tubule/sarcoplasmic reticulum junctions during development of excitation–contraction coupling in skeletal muscle. Mol. Biol. Cell. 1994;5:1105–1118. doi: 10.1091/mbc.5.10.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Protasi F. Ryanodine receptors of striated musclesa complex channel capable of multiple interactions. Physiol. Rev. 1997;77:699–729. doi: 10.1152/physrev.1997.77.3.699. [DOI] [PubMed] [Google Scholar]
- Giannini G., Clementi E., Ceci R., Marziali G., Sorrentino V. Expression of a ryanodine receptor-Ca2+ channel that is regulated by TGF-beta. Science. 1992;257:91–94. doi: 10.1126/science.1320290. [DOI] [PubMed] [Google Scholar]
- Giannini G., Conti A., Mammarella S., Scrobogna M., Sorrentino V. The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J. Cell Biol. 1995;128:893–904. doi: 10.1083/jcb.128.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko A., McKemy D.D., Kenyon J.L., Airey J.A., Sutko J.L. Embryonic chicken skeletal muscle cells fail to develop normal excitation–contraction coupling in the absence of the alpha ryanodine receptor. J. Biol. Chem. 1995;270:4220–4223. doi: 10.1074/jbc.270.9.4220. [DOI] [PubMed] [Google Scholar]
- Jeyaknmar L.H., Copello J.A., O'Malley A.M., Grassucci R., Wagenknecht T., Fleischer S. Purification and characterization of ryanodine receptor 3 from mammalian tissue. J. Biol. Chem. 1998;273:16011–16020. doi: 10.1074/jbc.273.26.16011. [DOI] [PubMed] [Google Scholar]
- Jorgensen A.O., Arnold W., Pepper D.R., Kahl S.D., Mandel F., Campbell K.P. A monoclonal antibody to the Ca2+-ATPase of cardiac sarcoplasmic reticulum cross-reacts with slow type I but not with fast type II canine skeletal muscle fibersan immunocytochemical and immunochemical study. Cell Motil. Cytoskelet. 1988;9:164–174. doi: 10.1002/cm.970090208. [DOI] [PubMed] [Google Scholar]
- Jorgensen A.O., Shen A.C., Arnold W., McPherson P.S., Campbell K.P. The Ca2+-release channel/ryanodine receptor is localized in junctional and corbular sarcoplasmic reticulum in cardiac muscle. J. Cell Biol. 1993;120:969–980. doi: 10.1083/jcb.120.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks A.R., Tempst P., Hwang K.S., Taubman M.B., Inui M., Chadwick C., Fleischer S., Nadal-Ginard B. Molecular cloning and characterization of the ryanodine receptor/junctional channel complex cDNA from skeletal muscle sarcoplasmic reticulum. Proc. Natl. Acad. Sci. 1989;USA. 86:8683–8687. doi: 10.1073/pnas.86.22.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W., Herrmann-Frank A., Lüttgau H.C. The role of Ca2+ ions in excitation–contraction coupling of skeletal muscle fibres. Biochim. Biophys. Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Morton M.E., Froehner S.C. Monoclonal antibody identifies a 200-kD a subunit of the dihydropyridine-sensitive calcium channel. J. Cell Biol. 1987;262:11904–11907. [PubMed] [Google Scholar]
- Murayama T., Ogawa Y. Characterization of type 3 ryanodine receptor (RyR3) in mammalian diaphragm muscle. J. Biol. Chem. 1997;272:24030–24037. doi: 10.1074/jbc.272.38.24030. [DOI] [PubMed] [Google Scholar]
- Nakai J., Imagawa T., Hakamata Y., Shigekawa M., Takeshima H., Numa S. Primary structure and functional expression from cDNA of the cardiac ryanodine receptor/calcium release channel. FEBS Lett. 1990;271:169–177. doi: 10.1016/0014-5793(90)80399-4. [DOI] [PubMed] [Google Scholar]
- Nakai J., Dirksen R.T., Nguyen H.T., Pessah I.N., Beam K.G., Allen P.D. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996;380:72–75. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- O'Brien J., Meissner G., Block B.A. The fastest contracting muscles of nonmammalian vertebrates express only one isoform of the ryanodine receptor. Biophys. J. 1993;65:2418–2427. doi: 10.1016/S0006-3495(93)81303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu K., Willard H.F., Khanna V.K., Zorzato F., Green N.M., MacLennan D.H. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J. Biol. Chem. 1990;265:13472–13483. [PubMed] [Google Scholar]
- Ottini L., Marziali G., Conti A., Charlesworth A., Sorrentino V. Alpha and beta isoforms of ryanodine receptor from chicken skeletal muscle are the homologues of mammalian RyR1 and RyR3. Biochem. J. 1996;315:207–216. doi: 10.1042/bj3150207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyamada H., Murayama Y., Takagi T., Iino M., Iwabe N., Miyata T., Ogawa Y., Endo M. Primary structure and distribution of ryanodine-binding protein isoforms of the bullfrog skeletal muscle. J. Biol. Chem. 1994;269:17206–17214. [PubMed] [Google Scholar]
- Powell J.A., Petherbridge L., Flucher B.E. Formation of triads without the dihydropyridine receptor a subunits in cell lines from dysgenic skeletal muscle. J. Cell Biol. 1996;134:375–387. doi: 10.1083/jcb.134.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protasi F., Franzini-Armstrong C., Flucher B.E. Coordinated incorporation of skeletal muscle dihydropyridine receptors and ryanodine receptors in peripheral couplings of BC3H1 cells. J. Cell Biol. 1997;137:859–870. doi: 10.1083/jcb.137.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios E., Pizarro G., Stefano E. Charge movement and the nature of the signal transduction in skeletal muscle excitation–contraction coupling. Ann. Rev. Physiol. 1992;54:109–133. doi: 10.1146/annurev.ph.54.030192.000545. [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Gorza L., Pitton G., Sagin L., Ausoni S., Sartore S., Lumo T. Embryonic and neonatal myosin heavy chain in denervated and paralyzed rat skeletal muscle. Dev. Biol. 1988;127:1–11. doi: 10.1016/0012-1606(88)90183-2. [DOI] [PubMed] [Google Scholar]
- Sonnleitner A., Conti A., Bertocchini F., Schindler H., Sorrentino V. Functional properties of the Ryanodine receptor type 3 (RyR3) Ca2+ release channel. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:2790–2798. doi: 10.1093/emboj/17.10.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino, V. 1995. Ryanodine Receptors. V. Sorrentino, editor. CRC Press, Inc., Boca Raton, FL.
- Sorrentino V., Reggiani C. Expression of ryanodine receptor type 3 in skeletal musclea new partner in excitation–contraction coupling? Trends Cardiovasc. Med. 1999;9:53–60. doi: 10.1016/s1050-1738(99)00003-1. [DOI] [PubMed] [Google Scholar]
- Stern M., Pizarro G., Rios E. Local control model of excitation–contraction coupling in skeletal muscle. J. Gen. Physiol. 1997;110:415–440. doi: 10.1085/jgp.110.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko J.L., Airey J.A. Ryanodine receptor Ca2+ release channelsdoes diversity in form equal diversity in function? Physiol. Rev. 1996;76:1027–1071. doi: 10.1152/physrev.1996.76.4.1027. [DOI] [PubMed] [Google Scholar]
- Sutko J.L., Airey I.A., Murakami K., Takeda M., Beck C., Deerinck T., Ellisman M.H. Foot protein isoforms are expressed at different times during embryonic chick skeletal muscle development. J. Cell. Biol. 1991;113:793–803. doi: 10.1083/jcb.113.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekura H., Nishi M., Noda T., Takeshima H., Franzini-Armstrong C. Abnormal junctions between surface membrane and sarcoplasmic reticulum in skeletal muscle with a mutation targeted to the ryanodine receptor. Proc. Natl. Acad. Sci. USA. 1995;92:3381–3385. doi: 10.1073/pnas.92.8.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989;339:439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Iino M., Takekura H., Nishi M., Kuno J., Minowa O., Takano H., Noda T. Excitation–contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature. 1994;369:556–559. doi: 10.1038/369556a0. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Yamazawa T., Ikemoto T., Takekura H., Nishi M., Noda T., Iino M. Ca2+-induced Ca2+ release in myocytes from dyspedic mice lacking the type-1 ryanodine receptor. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:2999–3006. doi: 10.1002/j.1460-2075.1995.tb07302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H., Ikemoto T., Nishi M., Nishiyama N., Shimuta M., Sugitani Y., Kuno J., Saito I., Saito H., Endo M. Generation and characterization of mutant mice lacking ryanodine receptor type 3. J. Biol. Chem. 1996;271:19649–19652. doi: 10.1074/jbc.271.33.19649. [DOI] [PubMed] [Google Scholar]
- Tarroni P., Rossi D., Conti A., Sorrentino V. Expression of the ryanodine receptor type 3 calcium release channel during development and differentiation of mammalian skeletal muscle cells. J. Biol. Chem. 1997;272:19808–19813. doi: 10.1074/jbc.272.32.19808. [DOI] [PubMed] [Google Scholar]
- Yamazawa T., Takeshima H., Sakurai T., Endo M., Iino M. Subtype specificity of the ryanodine receptor for Ca2+ signal amplification in excitation–contraction coupling. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:6172–6177. [PMC free article] [PubMed] [Google Scholar]
- Zorzato F., Fujii J., Otsu K., Phillips M., Green N.M., Lai F.A., Meillner G., MacLennan D.H. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1990;265:2244–2256. [PubMed] [Google Scholar]