Abstract
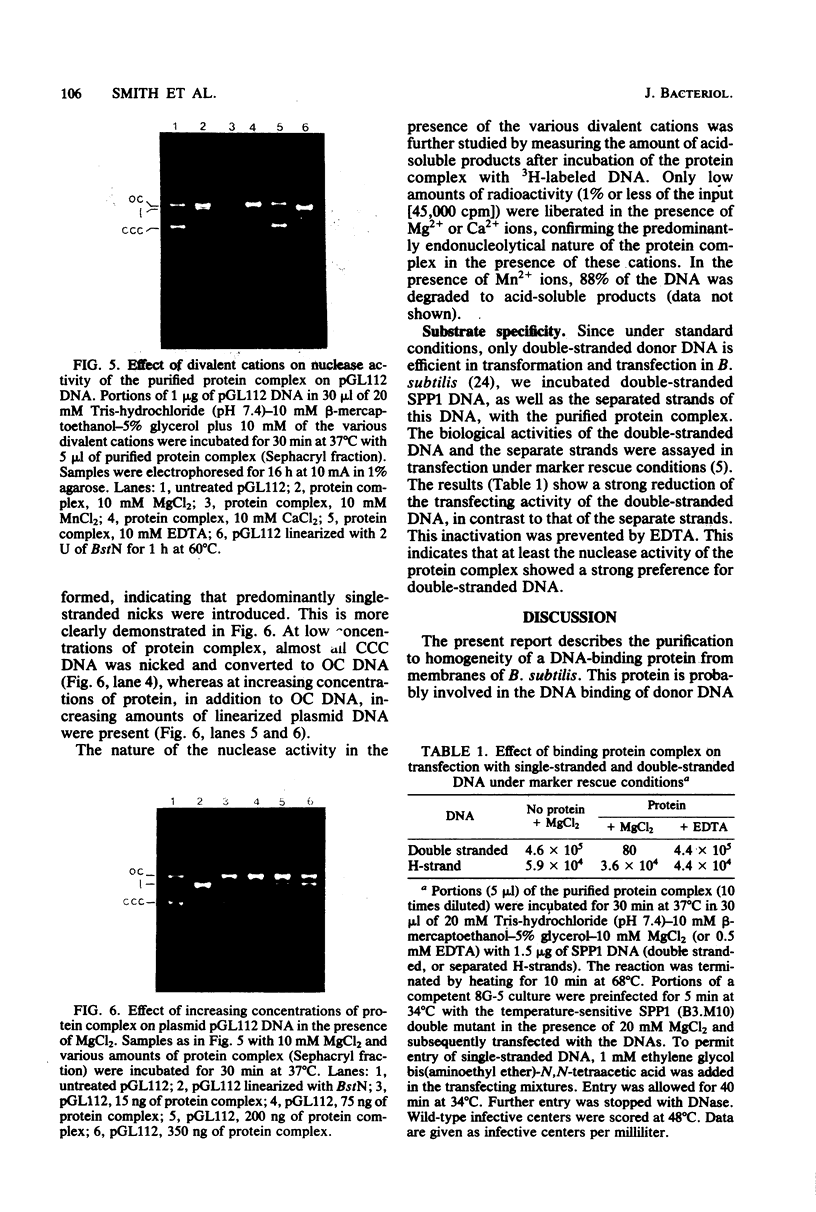
In DNA binding-deficient mutants of Bacillus subtilis a competence-specific protein with a subunit molecular weight of 18,000 was absent. The native protein containing this subunit was purified from B. subtilis membranes by chromatography on hydroxyapatite, DEAE-cellulose, and Sephacryl S-200. This protein appeared to be complexed with a second protein of slightly lower molecular weight (17,000) and a different isoelectric point. The native protein complex (apparent molecular weight, 75,000) contained approximately equal amounts of the two polypeptides and showed a strong DNA-binding activity. Incubation of the complex with plasmid and bacteriophage DNA revealed nuclease activity, specifically directed toward double-stranded DNA. Predominantly single-stranded nicks and a limited number of double-stranded breaks were introduced in the presence of Mg2+ ions. In the presence of Mn2+ ions the complex produced low-molecular-weight breakdown products from the DNA.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biswal N., Kleinschmidt A. K., Spatz H. C., Trautner T. A. Physical properties of the DNA of bacteriophage SP50. Mol Gen Genet. 1967;100(1):39–55. doi: 10.1007/BF00425774. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bron S., Luxen E., Venema G. Restriction and modification in Bacillus subtilis: effects on transfection under marker rescue conditions. J Virol. 1982 May;42(2):357–364. doi: 10.1128/jvi.42.2.357-364.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron S., Luxen E., Venema G., Trautner T. A. Restriction and modification in B. subtilis: effects on transformation and transfection with native and single-stranded DNA. Mol Gen Genet. 1980;179(1):103–110. doi: 10.1007/BF00268451. [DOI] [PubMed] [Google Scholar]
- Bron S., Murray K., Trautner T. A. Restriction and modification in B. subtilis. Purification and general properties of a restriction endonuclease from strain R. Mol Gen Genet. 1975 Dec 30;143(1):13–23. doi: 10.1007/BF00269416. [DOI] [PubMed] [Google Scholar]
- Bron S., Venema G. Ultraviolet inactivation and excision-repair in Bacillus subtilis. I. Construction and characterization of a transformable eightfold auxotrophic strain and two ultraviolet-sensitive derivatives. Mutat Res. 1972 May;15(1):1–10. doi: 10.1016/0027-5107(72)90086-3. [DOI] [PubMed] [Google Scholar]
- Canosi U., Morelli G., Trautner T. A. The relationship between molecular structure and transformation efficiency of some S. aureus plasmids isolated from B. subtilis. Mol Gen Genet. 1978 Nov 9;166(3):259–267. doi: 10.1007/BF00267617. [DOI] [PubMed] [Google Scholar]
- Davidoff-Abelson R., Dubnau D. Conditions affecting the isolation from transformed cells of Bacillus subtilis of high-molecular-weight single-stranded deoxyribonucleic acid of donor origin. J Bacteriol. 1973 Oct;116(1):146–153. doi: 10.1128/jb.116.1.146-153.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B., Neuberger M. Identification of a deoxyribonuclease implicated in genetic transformation of Diplococcus pneumoniae. J Bacteriol. 1975 Jul;123(1):222–232. doi: 10.1128/jb.123.1.222-232.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Single-strand breakage on binding of DNA to cells in the genetic transformation of Diplococcus pneumoniae. J Mol Biol. 1976 Feb 25;101(2):255–275. doi: 10.1016/0022-2836(76)90376-4. [DOI] [PubMed] [Google Scholar]
- Lacks S., Neuberger M. Membrane location of a deoxyribonuclease implicated in the genetic transformation of Diplococcus pneumoniae. J Bacteriol. 1975 Dec;124(3):1321–1329. doi: 10.1128/jb.124.3.1321-1329.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. Uptake of circular deoxyribonucleic acid and mechanism of deoxyribonucleic acid transport in genetic transformation of Streptococcus pneumoniae. J Bacteriol. 1979 May;138(2):404–409. doi: 10.1128/jb.138.2.404-409.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Early intermediate state of transforming deoxyribonucleic acid during uptake by Bacillus subtilis. J Bacteriol. 1971 Oct;108(1):38–44. doi: 10.1128/jb.108.1.38-44.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Breakage prior to entry of donor DNA in Pneumococcus transformation. Biochim Biophys Acta. 1973 Apr 11;299(4):545–556. doi: 10.1016/0005-2787(73)90226-8. [DOI] [PubMed] [Google Scholar]
- Mulder J. A., Venema G. Isolation and partial characterization of Bacillus subtilis mutants impaired in DNA entry. J Bacteriol. 1982 Apr;150(1):260–268. doi: 10.1128/jb.150.1.260-268.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J. A., Venema G. Transformation-deficient mutants of Bacillus subtilis impaired in competence-specific nuclease activities. J Bacteriol. 1982 Oct;152(1):166–174. doi: 10.1128/jb.152.1.166-174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. L., Lacks S. A. Complex structure of the membrane nuclease of Streptococcus pneumoniae revealed by two-dimensional electrophoresis. J Mol Biol. 1980 Aug 5;141(2):133–146. doi: 10.1016/0022-2836(80)90381-2. [DOI] [PubMed] [Google Scholar]
- Seto H., Tomasz A. Selective release of a deoxyribonucleic acid-binding factor from the surface of competent pneumococci. J Bacteriol. 1975 Nov;124(2):969–976. doi: 10.1128/jb.124.2.969-976.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. N. Number of deoxyribonucleic acid uptake sites in competent cells of Bacillus subtilis. J Bacteriol. 1972 Apr;110(1):266–272. doi: 10.1128/jb.110.1.266-272.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H., de Vos W., Bron S. Transformation in Bacillus subtilis: properties of DNA-binding-deficient mutants. J Bacteriol. 1983 Jan;153(1):12–20. doi: 10.1128/jb.153.1.12-20.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema G. Bacterial transformation. Adv Microb Physiol. 1979;19:245–331. doi: 10.1016/s0065-2911(08)60200-3. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]