Abstract
Cytoplasmic dynein is a multisubunit minus-end–directed microtubule motor that serves multiple cellular functions. Genetic studies in Drosophila and mouse have demonstrated that dynein function is essential in metazoan organisms. However, whether the essential function of dynein reflects a mitotic requirement, and what specific mitotic tasks require dynein remains controversial. Drosophila is an excellent genetic system in which to analyze dynein function in mitosis, providing excellent cytology in embryonic and somatic cells. We have used previously characterized recessive lethal mutations in the dynein heavy chain gene, Dhc64C, to reveal the contributions of the dynein motor to mitotic centrosome behavior in the syncytial embryo. Embryos lacking wild-type cytoplasmic dynein heavy chain were analyzed by in vivo analysis of rhodamine-labeled microtubules, as well as by immu-nofluorescence in situ methods. Comparisons between wild-type and Dhc64C mutant embryos reveal that dynein function is required for the attachment and migration of centrosomes along the nuclear envelope during interphase/prophase, and to maintain the attachment of centrosomes to mitotic spindle poles. The disruption of these centrosome attachments in mutant embryos reveals a critical role for dynein function and centrosome positioning in the spatial organization of the syncytial cytoplasm of the developing embryo.
Keywords: mitosis, centrosomes, dynein, syncytial blastoderm, Drosophila
Cytoplasmic dynein is a minus-end–directed microtubule ATPase that consists of two heavy chains, each with a microtubule motor domain, and a collection of smaller subunit proteins (for review see Holzbaur and Vallee 1994; Gibbons 1995). The dynein complex belongs to an extended family of microtubule motor proteins that are involved in diverse intracellular transport processes. Since the identification of cytoplasmic microtubule motors, there has been considerable interest in their mitotic function and regulation. Due in part to the utilization of these protein complexes in multiple cellular processes, these studies have been difficult. However, more recent biochemical, molecular, and genetic characterizations of motor proteins are providing reagents in a number of experimental systems that should help to unravel the function of motors during mitotic cell division. In the case of dynein, several recent investigations have provided models for dynein acting as kinetochore motors, or in centrosome migration and spindle morphogenesis, or even in cytokinesis (for review see Karsenti 1991; Hyman and Karsenti 1996; Merdes and Cleveland 1997; Karki et al. 1998; Walczak et al. 1998).
The action of a dynein motor at the kinetochore during mitosis remains elusive. Immunolocalization of dynein to the kinetochores of tissue culture cells, combined with the analysis of kinetochore microtubule polarity, first suggested dynein's potential role in providing the force for chromosome movements on the mitotic spindle (Pfarr et al. 1990; Steuer et al. 1990). More recent reports of dynein and dynactin dynamics at the kinetochore suggest that these molecules may function to mediate microtubule binding at the kinetochore (Wordeman and Mitchison 1995; Echeverri et al. 1996; Desai et al. 1997; Dujardin et al. 1998; Faulkner et al. 1998; Karki et al. 1998; Starr et al. 1998; Walczak et al. 1998). Functional evidence that supports such a role for dynein is limited to the recent observation in Tetrahymena that micronuclear chromosomes fail to segregate in cell lines in which the cytoplasmic dynein gene, DYH1, is knocked out (Lee et al. 1999).
A role for cytoplasmic dynein in spindle morphogenesis is presently more compelling, but discrepancies in the evidence from different experimental systems still exist. In mammalian tissue culture cells, the role of cytoplasmic dynein in cell cycle–related functions has been analyzed in antibody-mediated, dynein knockout experiments (Vaisberg et al. 1993). These studies were the first to demonstrate that cells injected with dynein antibodies before or during mitosis result in prophase arrest, with unseparated centrosomes and monopolar spindles. Conversely, injection of antibodies during metaphase or anaphase produces relatively minor effects on the progression or completion of mitosis. In contrast, molecular genetic studies to investigate the contribution of dynactin to mitotic spindle function have provided somewhat different results. Dynactin is a multisubunit regulatory complex that acts in the dynein functional pathway and is proposed to target the dynein motor to specific organelles, including the mitotic spindle (Allan 1994; Vallee et al. 1995; Holleran et al. 1998). Overexpression of the p50-dynamitin subunit of dynactin in transient transfections of tissue culture cells also produces a mitotic arrest. In this case, rather than defects in centrosome separation, the arrest is characterized by the splitting and fragmentation of the spindle (Echeverri et al. 1996). Similarly, subsequent antibody injection experiments that targeted the elimination of the dynein intermediate chain in mammalian cells also failed to reveal aberrant behavior of centrosomes during prophase (Gaglio et al. 1997).
In vitro model systems can exploit similar immunodepletion strategies and have provided new insights into dynein function at centrosomes and spindle poles. Mitotic Xenopus egg extracts, which are depleted for dynein motor function, show defects in spindle morphology and the attachment of centrosomes to the spindle apparatus (Verde et al. 1991; Heald et al. 1996; Merdes et al. 1996). This work has emphasized that the bipolar arrangement of spindle microtubules is achieved in the absence of centrosomes and dynein function. Furthermore, these in vitro model studies have suggested two distinct roles for dynein during spindle assembly. Dynein is proposed to bundle microtubules into a focused spindle pole and to ensure that an association between the centrosome and spindle is maintained (Gaglio et al. 1997; Heald et al. 1997; Merdes and Cleveland 1997; Walczak et al. 1998).
Thus far, genetic analysis of dynein function in mitosis has come largely from the fungal systems. In these organisms dynein is not essential, but mutations do exhibit nonlethal defects during cell division. For example, in Saccharomyces cerevisiae, the cytoplasmic dynein heavy chain (Dhc)1 gene is not required for viability, yet null mutations in the gene disrupt proper cell growth, mitotic spindle orientation, and nuclear migration (Eshel et al. 1993; Li et al. 1993). While cytoplasmic dynein alone does not appear to be essential for viability in yeast, a disruption of anaphase chromosome segregation is apparent in cells that lack the Dhc and the kinesin-related gene products, Cin8p and Kip1p (Saunders et al. 1995). In filamentous fungal systems the function of dynein is also nonessential. Mutational analyses of dynein components in Aspergillus nidulans (Xiang et al. 1994, Xiang et al. 1995), as well as Neurospora crassa (Plamann et al. 1994; Bruno et al. 1996), have supported a role for dynein in nuclear migration, but not nuclear divisions. The recent analysis of dynein heavy chain mutations in Nectria haematococca have provided evidence for the action of dynein in microtubule assembly and/or stability at spindle pole bodies and resultant defects in spindle elongation (Inoue et al. 1998). Here again, the loss of dynein function has not been shown to be essential for mitotic spindle function. These fungal studies are in conflict with the perturbation of dynein function in animal cells and may reflect the evolution of distinct pathways for mechanisms of force production and spindle assembly during mitosis.
Unlike yeast and lower eukaryotes, dynein function in metazoan organisms is essential for viability. In both Drosophila melanogaster and Mus musculus, mutations in the cytoplasmic dynein heavy chain gene are recessive lethal. In addition to observations that indicate a developmental requirement for cytoplasmic dynein, our mutational analysis of dynein in Drosophila has provided evidence that the cytoplasmic motor is required for cell viability. However, whether this requirement reflects an essential action of dynein during mitosis has not been addressed previously in any metazoan organism. We have extended our analysis of dynein function in the present work to demonstrate that dynein is essential for mitotic divisions in Drosophila. We have capitalized on intragenic complementation of recessive lethal alleles to provide embryos that lack wild-type dynein heavy chain function. This strategy permits an analysis of dynein's mitotic function in a living embryo. Our studies of embryos that are compromised for dynein function reveal an unexpected role for dynein in the attachment of centrosomes to nuclei, as well as previously suspected functions in centrosome migration and the attachment of centrosomes to spindle poles. The loss of dynein function and defective centrosome behavior impacts the global organization of the syncytial cytoplasm as reflected in the loss of spindle autonomy and karyokinesis defects.
Materials and Methods
Drosophila Stocks
The isolation of Drosophila Dhc64C alleles used in this study has been described (Gepner et al. 1996). To analyze cytoplasmic dynein mutant phenotypes in syncytial blastoderm embryos, reciprocal crosses were made using the balanced Dhc64C mutant stocks mwh Dhc64C6-6 h st pp ss es/TM6B, D h Hu e ca and mwh jv Dhc64C6-8 ca/TM6B, D h Hu e ca. Transheterozygous Dhc64C6-6/Dhc64C6-8 progeny contained wild-type non-Dichaete wings and non-Humeral phenotypes. Dhc64C6-6/Dhc64C6-8 progeny were further identified by the characteristic recessive Dhc64C short thin bristle mutant phenotype previously reported for this and other viable transheterozygous dynein mutant combinations (Gepner et al. 1996). Analysis of maternal effect lethality was performed on embryos derived from mothers that contained Dhc64C6-6/Dhc64C6-8 that had been mated with wild-type Oregon R males for 3–4 d. Similarly, females mated with sibling Dhc64C6-6/Dhc64C6-8 males results in the maternal effect lethal phenotype. A wild-type dynein transgene, P{Dhc64CT} (McGrail and Hays 1997), was used to rescue all the dynein alleles used in this study. Hemizygous larvae derived from both hypomorphic dynein alleles Dhc64C6-10 and Dhc64C6-6 were used to examine mitosis within neuroblasts in situ (below). Df(3L)10H is a chromosomal deficiency which removes the entire Dhc64C gene (Gepner et al. 1996).
Embryo Preparation and Microinjection
Embryos from wild-type Oregon R or Dhc64C6-6/Dhc64C6-8 females were collected on agar culture media containing grape juice at 20–45-min intervals. In preparation for microinjection, embryos were dechorionated by hand and mounted onto glass 24 × 50-mm coverslips (#1 thickness) coated with a thin film of glue that was prepared by dissolving double-sided tape adhesive in heptane (Minden et al. 1989). Depending upon the relative humidity, embryos were briefly desiccated for 4–8 min using a covered dish containing anhydrous CaSO4. Prepared embryos were covered with oxygenated halocarbon oil (series 700; Halocarbon Products) and injected using a Narishige MN-151 apparatus attached to a Zeiss Axiovert microscope. The embryos were injected with mammalian brain tubulin conjugated with TRITC (Molecular Probes, Inc., or directly prepared by the method of Hyman 1991).
To address whether the observed phenotypes are specific to dynein dysfunction and not artifacts resulting from the microinjection of exogenous tubulin, we genetically introduced a tau-GFP chimeric transgene (kindly provided by Prof. Daniel St. Johnston, Cambridge, United Kingdom) into the background of Dhc64C6-6/Dhc64C6-8 animals. The expression of this chimeric transgene, driven by the maternally expressed Drosophila α-tubulin 67C promoter, provides an excellent marker for microtubules during early embryogenesis (Micklem et al. 1997).
Lethal Phase Determination
Virgin Dhc64C6-6/Dhc64C6-8 females were mated with wild-type Oregon R males for 3 d at 25°C on standard cornmeal media. To avoid embryo crowding and lethality due to anoxia, embryos were collected for up to 6 h at 3-h intervals on grape juice–agar media with agar plates at 25°C. After the collection of embryos, the total number of embryos was determined. At 30–36 h after egg lay, the number of hatched first instar larvae and empty chorions was determined. Subsequently, viable larvae were counted, transferred to glass vials containing cornmeal agar media, and incubated at 25°C. At 3–6 d of development at 25°C, the number of second and third instar larvae was determined. Similarly, the numbers of surviving third instar larvae, pupae, and adults were counted on days 8–13 of development.
Microscopy and Image Acquisition
Standard light microscopy was performed on a Zeiss Axioskop microscope equipped with both phase-contrast and DIC lenses. Images from embryos and larval brains were collected using a Bio-Rad MRC 600 or 1024 scanning confocal system mounted on a Nikon Diaphot 300 microscope equipped with a 15 mW krypton/argon laser. A 60×/1.4 NA Planapochromatic and objective lens was used for all analyses. Injections were made using a Narishige MN-151 injection apparatus attached to the microscope. After injection, embryos were previewed using epifluorescence to assess the success of the injections and to determine the developmental stages of injected embryos. Images were collected and saved either digitally to disk or directly to optical memory disk using a Panasonic model 2028 OMDR. Individual 640 × 480 pixel frames were collected at 3–6-s intervals using Bio-Rad COMOS or Lasersharp software time-lapse features at 2 Kalman filtering at normal speed settings, or slow scan mode. Digital files were processed in NIH Image. Individual still frames were saved as PICT or TIFF files and Adobe Photoshop was used to adjust image size and contrast and to crop and pseudocolor images. Prints were made using the Fujix Pictography 3000 and Tektronix Phaser 340 color printers.
Analysis of Centrosome Migration, Centrosome Attachment, and Nuclear Sizes
Series of time-lapse images were opened using custom macros and individual nuclei within an injected embryo were analyzed at the point nearest the time of nuclear envelope breakdown (NEBD). NEBD of all nuclei was not entirely synchronous in a single frame; thus, data were obtained by moving up or down frames within the series to determine the point of NEBD for an individual nucleus. Measurements were recorded for a given nucleus at NEBD. An X-Y center was determined for an individual nucleus using the select line tool and the angle tool was used to measure the angle between the separating centrosomes at the point of NEBD. The results of these measurements were analyzed and plotted using Microsoft Excel. The statistical significance of these measurements was determined using a t test module in Microsoft Excel.
The attachment of centrosomes to spindle poles was analyzed in fixed preparations by measuring the distance between a centrosome and the spindle pole to which it was attached (see Fig. 1). Centrosome position was established by determining the centroid of the γ-tubulin–stained foci in fixed preparations using NIH Image. The end of the spindle pole was defined by the position at which the α-tubulin fluorescence intensity in the half spindle narrowed to a minimum width at the pole. The distance between the centrosome and the spindle pole positions was determined using the NIH Image line tool and the data were analyzed using Microsoft Excel.
Figure 1.
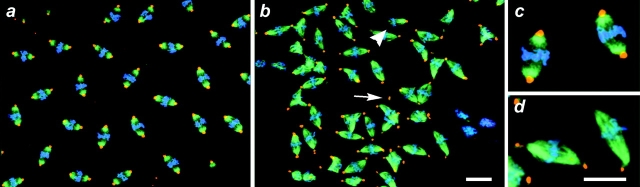
Mitotic defects are apparent in fixed mutant dynein Drosophila embryos. Shown are in situ confocal images of wild-type and Dhc64C mutant syncytial embryos. The mutant embryos were collected from Dhc64C6-8/Dhc64C6-6females, fixed, and prepared for immunofluorescence as described (see Materials and Methods). Shown are fields of metaphase nuclei for wild-type (a and c) and Dhc64C mutant (b and d) embryos at similar nuclear division cycles. c and d are enlargements of selected nuclei from a and b, respectively. The arrow points to a free centrosome and the arrowhead indicates a blunt-ended spindle lacking a centrosome. Note that the centrosomes at the periphery of a (e.g., upper left) attach to spindles at the edge of the embryo that tilt out of the image plane. DNA is pseudocolored blue, β-tubulin is green, and γ-tubulin is red. Bars, 10 μm.
Nuclear diameter measurements were accomplished in Image Pro Plus (Media Cybernetics) or NIH Image. Stacks of optical sections through the cortex region of four embryos for each genotype were collected. At the point in time nearest NEBD, maximum projections were made to determine the diameter of the nuclei from multiple focal planes. The dark areas corresponding to the nuclei were selected by density slicing and the nuclei were counted and measured. The resulting data were analyzed and plotted using Microsoft Excel. The statistical significance of these measurements was determined using a t test module in Microsoft Excel.
Preparation of Whole-Mount Embryos for Immunofluorescence
Embryos from dynein mutant Dhc64C6-6/Dhc64C6-8, Oregon R wild-type, or P{Dhc64CT}; Dhc64C6-6/Dhc64C6-8 (containing the wild-type Dhc64C transgene) were collected for up to 3 h and dechorionated using a 50% bleach solution. After dechorionation, embryos were rinsed in 0.1% Triton X-100 and fixed in heptane/methanol/EGTA (Hays et al. 1994). Fixed embryos were rehydrated for 5-min periods in a 70:30, 50:50, 30:70 PBS/methanol series followed by 5-min incubations in PBS and PBS containing 0.1% Triton X-100 (PBT). Before antibody labeling, embryos were blocked for 1 h at room temperature in PBS containing 0.1% Triton X-100, 1% BSA, and 0.02% sodium azide (PBT-BSA). All antibodies were diluted into PBT-BSA and incubations were performed at room temperature for up to 3 h or at 4°C for up to 18 h. After each antibody incubation, embryos were rinsed at 15–20-min intervals for 2–3 h in PBT-BSA at room temperature. Microtubules were labeled using a rat monoclonal anti–β-tubulin antibody (clone YOL1/34; Accurate Chemical Co.) diluted 1:10 and a Texas red–conjugated goat anti–rat secondary antibody (Jackson ImmunoResearch Labs). Centrosomes were labeled using a rabbit anti–γ-tubulin polyclonal antibody diluted 1:200 (kindly provided by Dr. Yixian Zheng, Carnegie Institute, Baltimore, MD) and a Cy-5 goat anti–rabbit (Amersham) or FITC-conjugated goat anti–rabbit secondary antibody (Boehringer Mannheim) diluted 1:100. For DNA labeling, embryos were treated with RNase (1 μg/ml) in PBS for 1 h at 37°C, then labeled with the nucleic acid probe Oligreen (1:200 in PBS) (Molecular Probes, Inc.) for 30 min at room temperature. After labeling, embryos were mounted in glycerol containing PBS and 1 mg/ml p-phenylenediamine and stored at −20°C.
Preparation of Larval Brain Whole-Mounts for Immunofluorescence
Third instar larval brains of the genotype Dhc64C6-10/Df(3L)10H and wild-type Oregon R were dissected in 0.7% saline and prepared for immunofluorescence according to published procedures (Gonzalez and Glover 1994). Microtubules were visualized with mouse anti–α-tubulin (Sigma T-9026) diluted 1:200 and Texas red–conjugated goat anti–mouse secondary antibody (Jackson ImmunoResearch Labs) diluted 1:200. Centrosomes were visualized with rabbit anti-CP190 (kindly provided by Dr. Will Whitfield, University of Dundee, Dundee, United Kingdom) diluted 1:250, and Cy-5 goat anti–rabbit (Amersham) diluted 1:200. DNA was stained with Sytox green (Molecular Probes, Inc.) diluted 1:1,500.
Results
Dynein Function Is Essential for Mitosis
To investigate the function of Dhc in mitosis we obtained animals that lack a functional Dhc and examined them in vivo. Using a collection of hypomorphic Dhc alleles in Drosophila, we obtained mutant animals that are compromised for dynein function at specific developmental stages or in specific tissues (McGrail et al. 1995; Gepner et al. 1996; Wojcik, E.J., and T.S. Hays, manuscript in preparation). We have identified heteroallelic combinations of Dhc64C that enable us to examine the role of dynein in mitosis during early embryogenesis. A combination of lethal hypomorphic alleles, Dhc64C6-6 and Dhc64C6-8, exhibits complementation for the zygotic requirement of dynein function, but results in maternal effect lethality.
Adults of the Dhc64C6-6/Dhc64C6-8genotype are recovered in equal proportion to nonmutant sibling classes, indicating that these heteroallelic adults are not less viable than wild-type animals. Dhc64C6-6/Dhc64C6-8 adult females lay eggs that lack wild-type dynein heavy chain before the onset of zygotic transcription. When Dhc64C6-6/Dhc64C6-8 adult females are mated to wild-type males (+/+), 94% of the resultant embryos fail to survive beyond the embryogenesis (Table ). Most of these embryos fail to properly cellularize and cannot complete gastrulation. Approximately 5.7% of the remaining embryos survive through hatching, with 1.4% of these embryos failing to complete larval development. The observed maternal effect lethality can be rescued by addition of one copy of a wild-type dynein transgene (McGrail and Hays 1997), which confirms that the maternal effect lethality results from loss of dynein function. The ∼3% of embryos that escape and complete larval development survive to adulthood. These escapers may be rescued by the initiation of zygotic expression of the paternally provided wild-type dynein heavy chain gene. While most transheterozygous Dhc64C6-6/Dhc64C6-8 embryos display early embryonic lethality, neither embryos containing a wild-type dynein transgene nor embryos derived from heterozygous adult females that carry a single mutant allele show dramatic reduction in viability with 96% or greater of these embryos surviving and completing development.
Table 1.
Analysis of Dhc64C6-6/Dhc64C6-8 Maternal Effect Lethality
| Lethal phase | n (dead) | Distribution of mortality |
|---|---|---|
| Embryos (24–36 h) | 1,039 | 94.2% |
| Larvae | 16 | 1.4% |
| Pupae | 15 | 1.3% |
| Adults | — | (33 viable adults: 3%) |
The failure to complete cellularization of the early embryo suggests that dynein plays a critical role in the early syncytial mitotic divisions and is consistent with clonal analysis demonstrating that dynein function is required for cell division and/or viability (Gepner et al. 1996). In the studies that follow, we have further characterized the maternal effect lethal phenotype to directly establish a requirement for cytoplasmic dynein in several aspects of syncytial mitotic divisions.
Mitotic Phenotypes in Fixed Preparations of Dynein Mutant Embryos
The majority of Dhc64C6-6/Dhc64C6-8 syncytial blastoderm embryos arrest in early embryogenesis before cellularization. We examined the fidelity of the syncytial mitotic divisions in situ within fixed whole-mount wild-type (Fig. 1, a and c) and mutant (Fig. 1b and Fig. d) specimens using confocal microscopy. Indirect immunofluorescence using antibodies that recognize γ-tubulin and β-tubulin, as well as the DNA dye Oligreen, was used to visualize the centrosome, mitotic apparatus, and chromosomes, respectively (Fig. 1). A range of abnormalities in the structural configurations of individual mitotic spindles within the syncytium was apparent in mutant embryos (Fig. 1b and Fig. d). In all studies, the defects were shown to be specific to mutant embryos by comparison to wild-type (Fig. 1, a and c) or sibling embryos that were processed for immunofluorescence in parallel.
Two predominant mitotic defects, free centrosomes and multipolar spindle arrays, were commonly found in fixed preparations of embryos that lack wild-type dynein function. First we will address our data concerning defective centrosome attachment. Free centrosomes can be found singly (Fig. 1 b) or in numbers (see Fig. 4 e). The origin of some of these centrosomes may be deduced from the presence of spindles lacking one or both centrosomes at their poles, and is suggestive of poor affinity between centrosomes and spindle microtubules in dynein mutant embryos. Corroborating evidence of a disrupted association between centrosomes and spindle poles was obtained from an analysis of γ-tubulin and β-tubulin distribution in the spindles of mutant and wild-type embryos (Fig. 1c and Fig. d). The γ-tubulin antigen (red) is generally restricted to the centrosome, while β-tubulin (green) is present in microtubules throughout the spindle. In wild-type embryos, the overlap in the immunolocalization of the two antigens reveals a tight association between the centrosomes and spindle poles (Fig. 1 c). Measurement of the distance between the center of γ-tubulin staining and the end of the spindle pole gave a mean value of 0.8 μm (number of poles measured = 164; SD = 0.05). In contrast, the centrosomes are not tightly associated with the ends of the fusiform spindle in the mutant embryos, but are visible as distinct foci displaced from the spindle pole (Fig. 1 d). The mean distance between centrosomes and the associated spindle poles in the mutant embryo was 1.8 μm (SD = 0.15; number of poles measured = 132) and is significantly different from wild-type (t stat = 2.83; 97% significance).
Figure 4.
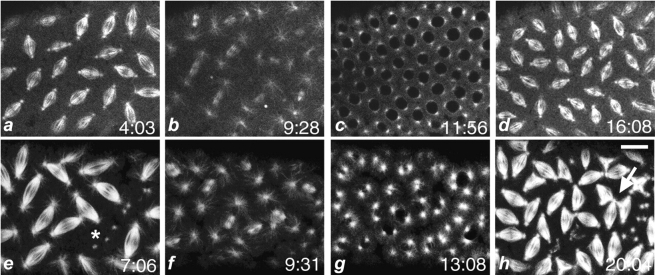
Dynein mutant embryos display mitotic defects. Time course of Drosophila wild-type and mutant syncytial embryos in vivo by confocal microscopy. The embryos were collected from wild-type Oregon R or Dhc64C6-8/Dhc64C6-6females and microinjected with TRITC-labeled tubulin. Shown are successive confocal images selected from time-lapse collections at the indicated time points (minutes). a–d follow a sequence of mitotic cycles in a representative wild-type embryo, and e–h follow a sequence of mitotic cycles in a typical mutant embryo. Shown is a field of metaphase spindles in a and e, telophase spindles in b and f, prophase spindles in c and g, and the subsequent metaphase in d and h. The arrow points to abnormal spindle interactions. The asterisk marks a patch of free centrosomes. Bar, 10 μm.
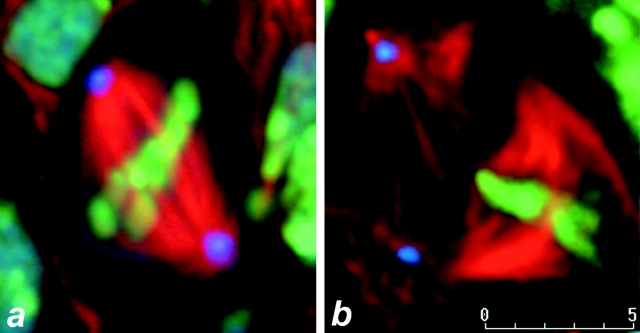
To test the relationship between dynein dysfunction and centrosome detachment from spindle poles, we examined mitosis in Drosophila larval neuroblasts from both wild-type and dynein mutants. The rapid Drosophila syncytial divisions, described above, undergo a cell cycle lacking in gap phases and take place within a unicellular environment. In contrast, the Drosophila central nervous system is a cellularized tissue that, unlike the abbreviated syncytial cell cycle, undergoes complex and patterned cell divisions (Foe and Alberts 1983). Mitotic neuroblasts from flies hemizygous for either of two independent lethal dynein alleles (Dhc64C6-10, Dhc64C6-6) were immunohistochemically examined in whole-mount fixed larval brains (Fig. 2). Significantly, a reduced affinity of centrosomes (blue) for spindle poles was frequently observed in the Dhc64C6-10and Dhc64C6-6 mutant lines (Fig. 2 b). In addition, spindle microtubule bundles were often disrupted and curved in the dynein mutant neuroblasts (Fig. 2 b). This phenotype was never observed in wild-type neuroblasts (Fig. 2 a). These defects are rescued by addition of wild-type dynein transgene. The above observations suggest that dynein function is required for the proper association between centrosomes and spindle poles during mitosis throughout Drosophila development.
Figure 2.
Dynein is required for centrosome attachment to spindle poles in larval neuroblasts. Confocal images of Drosophila wild-type and Dhc64C6-10/Df(3L)10H mutant larval neuroblasts taken from a fixed specimen (see Materials and Methods). Similar results were obtained for the genotype Dhc64C6-6/Df(3L)10H. Shown are optical slices of a metaphase neuroblast from wild-type (a) and mutant (b) larval brains. DNA is false-colored in green, α-tubulin is red, and CP190 centrosome antigen is blue. Bar, 5 μm.
A second class of mitotic defects that we noted in the fixed preparations of mutant embryos included multipolar spindle arrays. While rare in wild-type embryos, aberrant spindle configurations occurred at high frequency in the dynein mutants. Multipolar spindle arrays and bipolar spindles with aberrant numbers of centrosomes associated with each pole were abundant during nuclear cycles 10–13 in mutant embryos. In addition, spindle configurations frequently were excessively curved and the normally uniform spacing between spindles within the syncytium was disrupted (Fig. 1 b and 3 a). Multipolar microtubule arrays were judged to result from fusion of a number of neighboring spindles and associated chromatin (Fig. 1 b). In addition to multipolar spindles, we also observed abnormal spindles in which an apparently normal half-spindle containing a single centrosome, spindle pole and chromatin, was flanked by an abnormally blunt-ended pole lacking a detectable centrosome (Fig. 1 b, arrowhead). Significantly, these defects in spindle bipolarity (Fig. 3 a) and centrosome associations (Fig. 3 b) are also detected during very early nuclear cycles, well before cycle 10 and the migration of nuclei to form a closely packed monolayer within the cortical cytoplasm.
Figure 3.
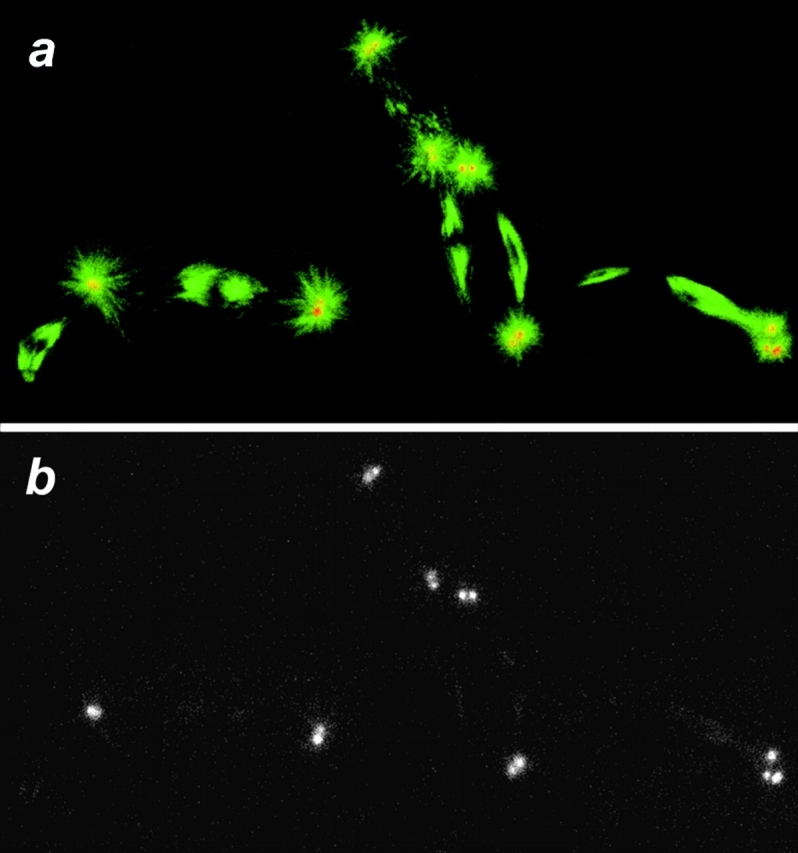
Mitotic defects can occur during the early nuclear cycles within dynein mutant embryos. Shown are in situ confocal images of mutant Drosophila syncytial metaphase nuclei. The image was taken from a cycle 3 embryo. The mutant embryos were collected from Dhc64C6-8/Dhc64C6-6females, fixed, and prepared for immunofluorescence as described (see Materials and Methods). a shows a field of mutant metaphase figures with β-tubulin false-colored in green and γ-tubulin in red (overlap appears orange). b shows only the γ-tubulin channel from a.
Dynein Dysfunction Results in Aberrant Centrosome Behavior during Mitosis in Living Embryos
To extend our understanding of how the mitotic phenotypes in fixed preparations arise, we analyzed syncytial mitotic divisions in living embryos. We visualized mitotic spindles after microinjecting rhodamine-labeled tubulin into living wild-type embryos and mutant embryos from Dhc64C6-6/Dhc64C6-8 mothers, and then recorded time-lapse movies of syncytial mitosis. After injection, wild-type embryos progressed normally through several rounds of mitosis (Fig. 4, a–d). Nuclear divisions were highly synchronous and proceeded in well-organized waves across the embryo. The orderly progression of nuclear cycles results in an evenly spaced monolayer of nuclei at the surface of the syncytial blastoderm. As noted by others (for example, Sullivan et al. 1990), we occasionally did observe nuclei that failed to complete mitosis. Such nuclei lose their association with the cell cortex and rapidly depart into the interior of the embryo; this event is termed “nuclear fallout.”
Unlike wild-type syncytial nuclear divisions, dynein mutant divisions progressed with poor synchrony and displayed profound defects in the behavior of the mitotic apparatus (Fig. 4, e–h). These defects occurred during any syncytial nuclear cycle with no discernible temporal or spatial pattern. In this regard, our in vivo study is entirely consistent with the analysis of dynein mutant phenotypes in fixed preparations of embryos. Moreover, the mutant phenotypes that we characterized in vivo using microinjection of rhodamine-labeled tubulin were also apparent when using a tau-GFP transgene to visualize microtubule arrays and dynamics (Micklem et al. 1997). This strategy circumvents the potential artifacts that may ensue after embryos undergo microinjection. The parallel observations made in fixed and living embryos substantiate that the observed defects are the consequence of dynein dysfunction. In presenting the results of our time-lapse analyses, we have divided the defective mitotic behaviors into four main categories: (1) abnormal centrosome migration; (2) pathways to the formation of free centrosomes; (3) pathways to multipolar spindle arrays; and (4) defects in karyokinesis.
Mutant Syncytia Display Abnormal Centrosome Migration.
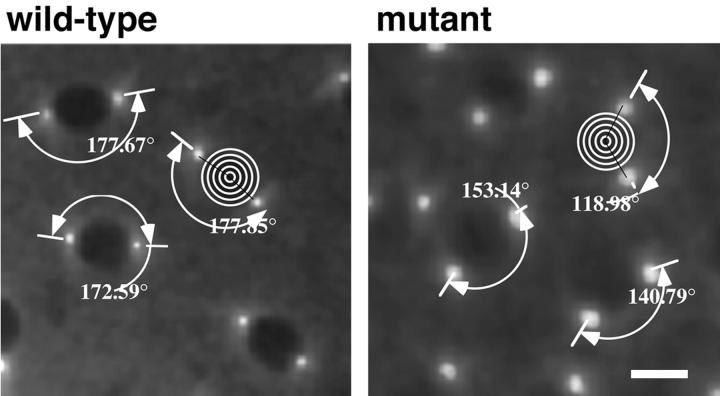
In fixed mutant specimens, we frequently observed the improper positioning of centrosomes off the spindle pole (Fig. 1 Fig. 2 Fig. 3). To determine a possible pathway to this condition, we examined centrosome separation and positioning in mutant embryos. A large fraction of the syncytial nuclei in mutant embryos failed to separate their centrosomes to a position fully 180° apart before the onset of NEBD. To quantify this defect we analyzed time-lapse records of mitosis in both mutant and wild-type embryos. In wild-type embryos, ∼98% of the centrosomes migrated around the nuclear envelope to final positions at NEBD to a mean displacement angle of between 170° and 180° (Fig. 5, wild-type). Only 2% of nuclei examined underwent less than 170° of separation and these typically were culled from the syncytial monolayer by nuclear fallout (Table ).
Figure 5.
Dynein is required for complete centrosome migration. The centrosome migration defect was measured in the manner indicated in both wild-type and dynein mutant embryos (see Materials and Methods). Prophase centrosomes were visualized in vivo by confocal microscopy after microinjection of TRITC-labeled tubulin. The wild-type panel shows a field of syncytial prophase nuclei and associated centrosome separation angles. The mutant panel shows a field of syncytial prophase nuclei and their centrosome separation angles in Dhc64C6-8/Dhc64C6-6embryos. Bar, 5 μm.
Table 2.
Analysis of Dhc64C6-6/Dhc64C6-8 Centrosome Migration Defects
| Embryo (maternal genotype) | n | Mean | SD | Nuclei with separation angles less than 120° that progress to abnormal spindle configurations |
|---|---|---|---|---|
| Wild-type Oregon R | 115 spindles | 174.02° | 6.38 | <1% (1/115) |
| 3 embryos | ||||
| Dhc64C6-6/Dhc64C6-8 | 113 spindles | 136.47° | 56.53 | 76% (86/113) |
| 5 embryos |
In contrast, we determined that the mean centrosome migration and separation angle in mutant embryos at the time of NEBD is 136.47° (Fig. 5, mutant; Table ). The aberrant migration of centrosomes in mutant embryos correlates with subsequent defects in the affected mitotic apparatus. For instance, nearly all nuclei that later gave rise to multipolar spindles were found to produce a mean centrosome migration angle of 119.68° at the preceding NEBD (Fig. 5). The most severe defects in centrosome separation concluded with a centrosome separation angle of 102.28° and were associated with the most aberrant spindle arrays, including bipolar and multipolar configurations that failed to progress through the mitotic cycle. However, nuclei that exhibited less extreme defects in centrosome separation and subsequent spindle assembly continued through the cycle. Thus, it is likely that a primary defect resulting from dynein dysfunction is the compromised migration of centrosomes.
Dynein Mutant Syncytial Embryos Accumulate Free Centrosomes by Several Pathways.
Analyses of time-lapse sequences were conducted to establish the origin of free centrosomes present in mutant embryos. We find that free centrosomes can arise during both early and late nuclear cycles by different pathways that are independent of cell cycle stage. First, we observed centrosomes departing the nuclear envelope during prophase in mutant embryos (Fig. 6). The affected nucleus, bearing only a single centrosome, would often attempt to complete the current mitotic cycle. Free centrosomes observed in the cortical layer persisted and replicated during the final nuclear cycles in synchrony with surrounding nuclei. We never observed this pathway in wild-type embryos.
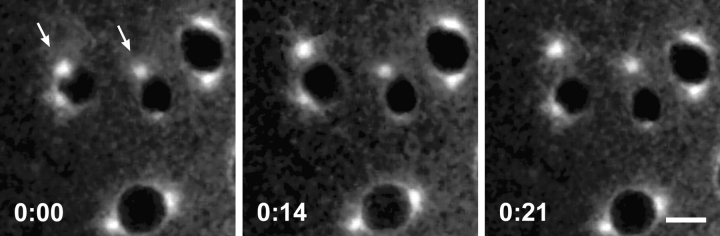
Figure 6.
Centrosomes can detach from the nuclear envelope in the dynein mutants. Time course of mitosis in a syncytial embryo provided by Dhc64C6-8/Dhc64C6-6females. Microtubules are labeled with TRITC-tubulin. Presented are selected in vivo confocal images that highlight nuclei and labeled centrosomes. The images were taken at the indicated time points (minutes) during prophase. Arrows point to centrosomes, which become separated from the nuclear envelope during the time course shown. Bar, 5 μm.
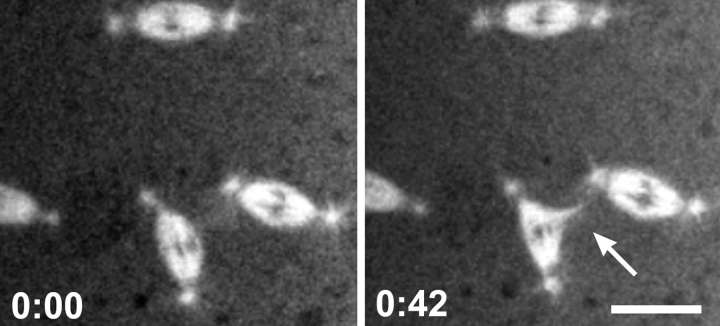
The detachment of centrosomes from bipolar and multipolar spindles was also observed. Relative to the loss of centrosomes from the nuclear envelope, the detachment of centrosomes from mitotic spindle poles was more frequently captured in a single focal plane during time-lapse imaging. Centrosome loss from bipolar spindles often resulted in the partial collapse of the affected spindle pole (Fig. 7 a). Such nuclei invariably failed to complete the current cycle successfully and dropped into the interior of the embryo. This result is supported by observations in fixed mutant embryos of normal bipolar spindles that lack a centrosome at one pole (Fig. 1 b). However, in the case of multipolar spindle configurations, the loss of a centrosome was always accompanied by the complete collapse of the microtubule array associated with the centrosome. Fig. 7 b shows a time-lapse sequence of a spindle that has four poles. The upper centrosome completely detaches from its spindle pole while the remaining three centrosomes appear to be tenuously associated with the spindle poles. In this example, the upper centrosome loses its association with the spindle pole and, subsequently, the associated spindle pole collapses (see also Fig. 10).
Figure 7.
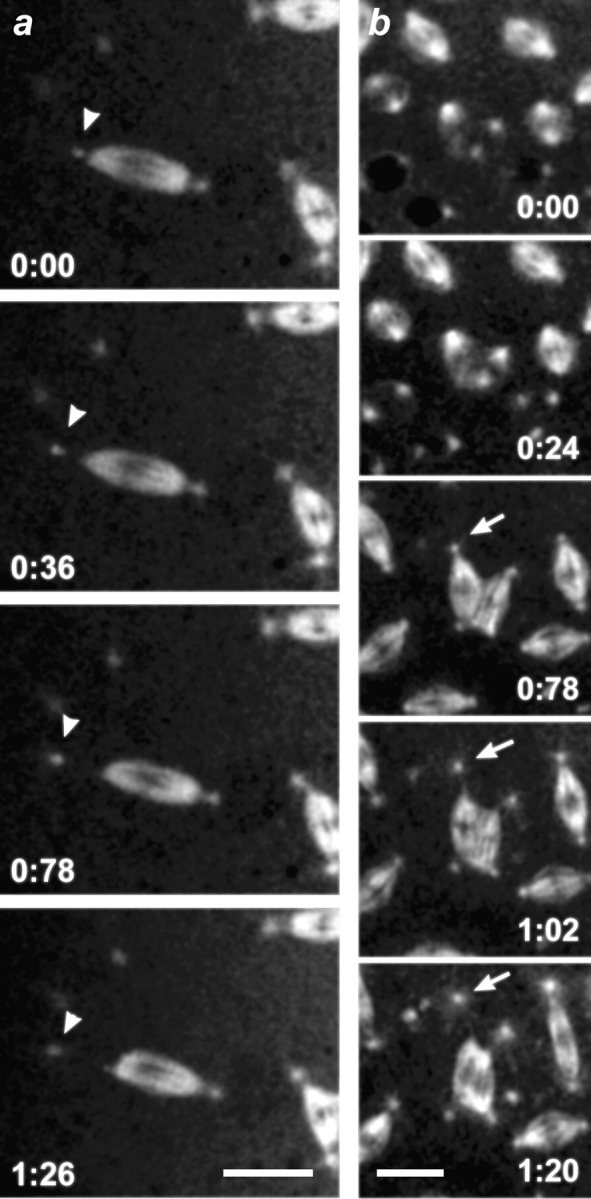
Centrosomes can detach from metaphase spindles in the dynein mutants. In vivo time course of mitosis during metaphase in a syncytial embryo provided by Dhc64C6-8/Dhc64C6-6females. Shown are selected confocal images of a field containing TRITC-labeled spindles and centrosomes at the indicated time points (minutes). (a) Centrosomes can detach from bipolar spindles. The arrowhead marks a centrosome which detaches and moves away from one pole of a bipolar spindle. (b) Centrosomes can detach from multipolar spindle arrays. The arrow points to a centrosome that detaches and moves away from a multipolar spindle. The associated spindle pole subsequently collapses. Bars, 10 μm.
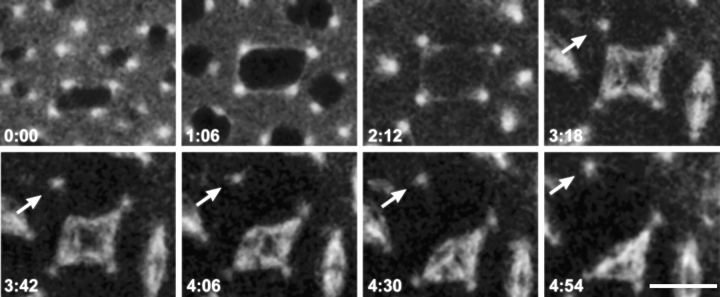
Figure 10.
Ectopic spindle pole formation in mutant dynein syncytial embryos. Time points (minutes) of metaphase during mitosis in an embryo provided by Dhc64C6-8/Dhc64C6-6females. Shown are two panels containing metaphase nuclei with spindles visualized with TRITC-tubulin at the time points indicated. The arrow points to an ectopic spindle pole generated between two spindles. The microtubule bundle composing the new pole has split away from the parent spindle. Bar, 10 μm.
A final pathway that contributes to the accumulation of free centrosomes in the dynein mutant embryos involves the removal of defective nuclei. Aberrant spindle configurations produce aberrant mitotic products that are eliminated during cycles 10–13 by nuclear fallout. As the defective nuclei drop from the cortex into the interior cytoplasm, the centrosomes associated with such nuclei remain in the cortical layer. This is likely to be the predominant mechanism that contributes to the patches of free centrosomes observed at the surface of embryos.
Pathways to the Formation of Multipolar Arrays.
The formation of multipolar arrays in mutant embryos produced by Dhc64C6-6/Dhc64C6-8 females most commonly occurred by the aberrant fusion of adjacent mitotic spindles in the syncytium. This event frequently correlated with the improper migration and separation of duplicated centrosomes (described above) before the assembly of the spindle. The fusion of neighboring spindles would often result in the formation of a bipolar or quadripolar structure which would fail to undergo anaphase (Fig. 8). Instead, such an array would often progress directly into interphase. The abnormal metaphase array would disassemble and reform a nuclear envelope with an aberrant number of associated centrosomes. At the next nuclear cycle, the presence of multiple centrosomes on a single nucleus would provide another pathway toward the formation of multipolar spindle arrays (Fig. 9). In addition to nuclei retaining aberrant numbers of centrosomes following an aborted mitosis, nuclei were also occasionally observed to capture a free centrosome in close proximity.
Figure 8.
Fusion of neighboring mitotic arrays in mutant dynein syncytial embryos. Time course of mitosis in vivo within an embryo provided by Dhc64C6-8/Dhc64C6-6females. Microtubules are labeled with TRITC-tubulin. Shown are selected confocal images of spindles at the indicated time points (minutes) during metaphase in vivo within a syncytial embryo. Two spindles (arrows) undergo fusion to form a single bipolar array. Bar, 10 μm.
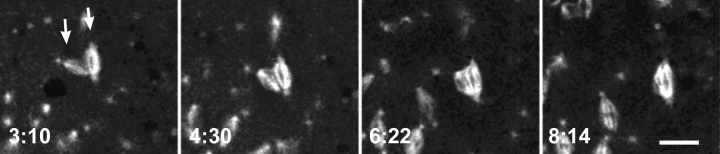
Figure 9.
Alternative pathway for the formation of multipolar spindle arrays. Time-lapse series of confocal images of nuclei in an embryo from Dhc64C6-8/Dhc64C6-6females. Microtubules are labeled with TRITC-labeled tubulin. Shown are syncytial nuclei at the indicated time points (minutes) during the prophase to metaphase transition. A prophase nucleus bearing four centrosomes develops into an abnormal tetrapolar mitotic spindle. A centrosome (arrows) can be seen to detach and move away from one of the poles. This pole subsequently collapses. Bar, 10 μm.
Multipolar configurations were also generated by the dominant influence of centrosomes on neighboring spindles in the syncytial cytoplasm. In the mutant embryos, spindle-associated or single free centrosomes were capable of inducing ectopic spindle poles on adjacent mitotic arrays. Fig. 10 shows such an example where the resident centrosome of one spindle interacts with a bipolar spindle array in close proximity. In this case, an ectopic spindle pole is formed when a bundle of microtubules is split off from the bipolar spindle and becomes focused towards the neighboring centrosome. We never observed such activity by centrosomes of closely opposed spindles in wild-type embryos. These results suggest that the reduced affinity of centrosomes for spindle poles in mutant embryos can promote inappropriate interactions between centrosomes and microtubule arrays.
Mutant Nuclei Exhibit Defects in Karyokinesis.
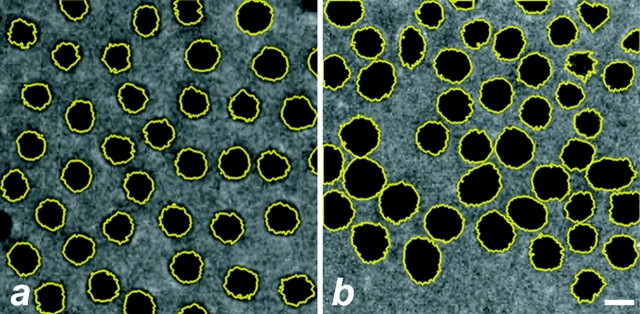
An additional defect in chromosome segregation is suggested by the variable size of interphase nuclei in mutant embryos. Z-series confocal optical sections were collected through the cortical layer of nuclei in late stage syncytial embryos and maximum projections were made to determine nuclear diameters. As shown in Fig. 11, nuclei in mutant embryos showed a nearly twofold greater range in nuclear diameters relative to nuclei in wild-type embryos. Because the relative size of syncytial nuclei is held to be indicative of DNA content, such variation indicates that karyokinesis in dynein mutants is defective. The analysis of optical sections that extended through the cortical layer and into the interior of mutant embryos also revealed a nonuniform spacing of nuclei, as well as frequent patches at the surface that were devoid of nuclei. Such defects in the cortical monolayer of nuclei were never observed in wild-type embryos. As previously proposed, a mechanism of nuclear fallout may serve to remove aberrant nuclei that result from defective mitosis in dynein mutant embryos.
Figure 11.
Nuclear DNA content is affected in the dynein mutant syncytial nuclei. Shown is an example of the method used (see Materials and Methods) to estimate the relative nuclear volume of both wild-type (a) and dynein mutant (b) Drosophila embryos. Syncytial nuclei are outlined in yellow and false-colored in black. Note variable size and spatial distribution of nuclei in the Dhc mutant embryo. The average diameters of wild-type and dynein mutant nuclei are 11.48 ± 2.58 μm (4 embryos, 428 nuclei), and 13.75 ± 4.47 μm (4 embryos, 437 nuclei), respectively. Bar, 5 μm. The difference in nuclear size is significant (t stat = 3.93; 96% significance).
Discussion
Dynein Is Essential for Mitosis in Drosophila
In this study, we identified recessive lethal alleles of the dynein heavy chain gene, Dhc64C, that exhibit intragenic complementation and reveal mitotic phenotypes during the rapid divisions of the syncytial embryo. The nonlethal Dhc64C6-8/Dhc64C6-6combination of mutations results in fully viable transheterozygous adult females that produce eggs that are endowed with strictly mutant dynein heavy chain. The rapid rounds of nuclear divisions that follow fertilization of these mutant embryos are compromised by the defective dynein and result in maternal effect lethality with >94% of the embryos dying. The nature of the lesions within the dynein heavy chain mutations, Dhc64C6-8and Dhc64C6-6, are not known. However, the presence of a wild-type dynein heavy chain transgene rescues the mitotic defects, as well as maternal effect lethality, demonstrating that the phenotype is specific to a loss in dynein function. Importantly, the mitotic defects we uncovered are not unique to the syncytial nature of early nuclear divisions. We discovered similar defects occurring in the larval neuroblasts of late-lethal alleles of the dynein heavy chain.
Within the mutant syncytium, nuclear cycles proceed and repeatedly show defects in specific centrosome behaviors and spindle morphogenesis at each nuclear cycle. This progression of the nuclear cycles and the repetitive occurrence of centrosome misbehavior and aberrant multipolar spindle formation is consistent with the defects being a primary consequence of dynein dysfunction. In this regard, we suggest that the combination of hypomorphic heavy chain alleles provides a means to specifically attenuate dynein function in order to investigate its mitotic function in early syncytial embryos. Strong loss of function alleles or null mutations in the dynein heavy chain are cell lethal and prohibit such analysis. This result contrasts with findings in other genetically tractable systems, such as yeast, in which it has been shown that dynein is not an essential gene (Eshel et al. 1993; Li et al. 1993; Saunders et al. 1995). Our results demonstrate that the mitotic function(s) of cytoplasmic dynein are essential in Drosophila.
Dynein Is Required for Nuclear Attachment and Migration of Centrosomes
Analysis of centrosome behavior in vivo within the dynein mutant embryos occasionally revealed centrosomes detaching from the nuclear envelope. In time-lapse movies some centrosomes left the envelope never to return, while other centrosomes detached briefly and then moved back to the nucleus and reattached. These events are never seen in wild-type embryos and provide evidence for a novel function for dynein in maintaining the association of centrosomes with the nuclear envelope. The reattachment of centrosomes, as well as the low penetrance of the detached centrosome phenotype, is consistent with the prediction that the hypomorphic dynein gene products are only partially compromised for nuclear attachment. One interpretation of these phenotypes is that dynein is associated with the nuclear envelope, where it acts as a minus-end motor to draw in centrosomal microtubules and secure the centrosomes to the nuclear membrane. Alternatively, or in addition, dynein may reside in the centrosome and act to stabilize the attachment of nucleated microtubules that are themselves required for nuclear attachment. In this case, loss of dynein function may increase the frequency of microtubule release from centrosomes (Keating et al. 1997; Waterman-Storer et al. 1997) and thus weaken nuclear attachment. Evidence for active dynein complex associated with the nuclear envelope has been reported in vitro in Xenopus extracts (Reinsch and Gonczy 1998). In Drosophila we have previously noted that cytoplasmic dynein is localized to the oocyte nucleus, where it might power nuclear migration (Li et al. 1994). However, in embryos, dynein is present on the mitotic spindle and appears concentrated at spindle poles, but no accumulation on nuclear envelopes has yet been detected (Hays et al. 1994; McGrail, M., unpublished data). In mammalian cell lines, dynein is localized to kinetochores, centrosomes, and at the nuclear periphery (Pfarr et al. 1990; Steuer et al. 1990; Busson et al. 1998).
Most centrosomes observed in mutant embryos retained their nuclear attachments, but exhibited defects in migration along the nuclear membrane during prophase. Our time-lapse analysis in living embryos demonstrates that dynein is required for the initial separation of centrosomes along the nuclear envelope and is distinct from the function of antagonistic motors that maintain the separation of centrosomes once initial separation is complete (Sharp et al. 1999). We frequently observe a failure of the duplicated centrosomes to fully migrate along the nuclear envelope to a position 180° apart before NEBD. The centrosome migration defect is consistent with results from antibody knockout experiments performed in a vertebrate cell culture system (Vaisberg et al. 1993). The predominant defect in centrosome migration can be viewed as an intermediate phenotype, the consequence of only partial loss of dynein function. The “detached-centrosome” phenotype might occur when the same dynein-based mechanism is further compromised. However, whether dynein function in centrosome attachment and centrosome migration are mechanistically related remains to be determined. Indeed, recent studies show that centrosome migration in Xenopus extracts depends upon the activity of the plus-end directed kinesin-like protein Xklp2 (Boleti et al. 1996). Xklp2 is a member of the BimC class of conserved kinesin-like proteins which likely play similar roles in several different eukaryotes (for review see Kashina et al. 1997). Furthermore, the localization of a COOH-terminal fragment of Xklp2 to the minus-ends of spindle and astral microtubules requires the activity of cytoplasmic dynein (Wittmann et al. 1998). Mutations in dynein may affect centrosome separation by reducing or preventing the normal accumulation of BimC class motor proteins to astral and spindle microtubules.
An opposing category of models for dynein involvement in centrosome separation predicts that force production occurs within the cortical cytoplasm and acts to pull on centrosomal microtubules. While such a model readily explains the centrosome migration defect, this mechanism does not account for the observed detachment of centrosomes.
Centrosome Attachment to Focused Spindle Poles Requires Dynein Function
In dynein mutant embryos, we observed the release of centrosomes from spindle poles, as well as “loosely attached” centrosomes, where the distance between a centrosome and the associated metaphase spindle pole is significantly greater than in wild-type. Furthermore, the morphology of the spindle poles which lose a centrosome is affected in a manner consistent with current models of dynein function in spindle morphogenesis (see Gaglio et al. 1997; Heald et al. 1997; Merdes and Cleveland 1997). In mutant embryos the detachment of a centrosome from bipolar spindles results in the partial collapse of the affected pole. In some cases, the blunt-ended pole becomes refocused, suggesting that either a residual function of the mutant dynein or an additional minus-end motor can rescue the spindle pole. Loss of a centrosome from multipolar spindles also results in collapse of the affected pole. Our observations demonstrate in living embryos that the maintenance of a focused spindle pole requires dynein and the stabilizing influence of a centrosome. This result is not contingent upon the syncytial environment of the embryo since a similar requirement for centrosomes in the organization of spindle poles is apparent in Drosophila larval neuroblasts.
Relationship between Centrosome Behavior and Spindle Morphogenesis
Our in vivo time-lapsed analysis provides a temporal understanding of the relationship between centrosome behavior and spindle morphogenesis, and reveals another novel aspect to the dynein mutant phenotype. We find that nuclei which undergo incomplete centrosome migration are predisposed to suffer further defects in spindle assembly that frequently lead to multipolar spindle configurations. As a further consequence, the size of interphase nuclei is variable and suggests a significant defect in karyokinesis. Although dynein is likely to be present on Drosophila kinetochores (Starr et al. 1998), as it is in other organisms (Pfarr et al. 1990; Steuer et al. 1990), evidence for a direct role for dynein in chromatid congression or segregation is lacking. The alignment of chromosomes at metaphase is apparently normal in mutant embryos and we favor the interpretation that aberrant nuclear size results predominantly from abnormal chromatid segregation on multipolar spindles, rather than a direct effect on kinetochore-mediated chromatid movement.
How does a loss in dynein function and defective centrosome behavior lead to multipolar spindle assembly? It previously has been shown that the regular spacing between metaphase spindles in the Drosophila syncytium is dependent upon centrosome positions on the nuclear envelope before M phase (Valdes-Perez and Minden 1995). One interesting possibility is that organization of the cortical cytoskeleton and the pseudocleavage furrow acts to help isolate nuclei from one another during late nuclear division cycles and is disrupted by mispositioned centrosomes. In this case, loss of spindle autonomy and the formation of multipolar spindle configurations is an indirect effect of the role of dynein in centrosome positioning. However, mutations known to disrupt the cortical cytoskeleton and pseudocleavage furrows are reported to promote spindle fusions during late nuclear cycles when nuclear density is high (Sullivan et al. 1990; Callaini et al. 1992; Postner et al. 1992). For dynein mutant embryos, spindle fusions are detected during the earliest rounds of division before cortical migration and when nuclear density is quite low.
Alternatively, a reduction in dynein function in mitotic spindles and/or the syncytial cytoplasm may allow spindle or centrosomal microtubule bundles to interact inappropriately with neighboring arrays. Measurements of an increase in spindle girth in the mutant embryos is consistent with a reduced organization of microtubules within mutant spindles (Sanders, M.A., unpublished data). In spite of the reduced affinity of centrosomes for both nuclear envelopes and spindle poles in the dynein mutants, centrosomes retain a strong capacity to organize spindle poles. We have observed ectopic spindle pole formation on neighboring mitotic arrays by both free centrosomes and spindle-associated centrosomes. The ectopic poles form by splitting off bundles of microtubules from the adjacent spindle, rather than by nucleation of microtubule bundles from the errant centrosome toward the adjacent spindle. Subsequently, the formation of interspindle microtubule bundles and the fusion of neighboring spindles may result from the action of other motor activities. For example, it was recently shown that the separation of spindles during late nuclear cycles in Drosophila embryos requires the function of KLP61F (Sharp et al. 1999).
In summary, we have provided the first evidence that cytoplasmic dynein is required for the attachment of centrosomes to prophase nuclei. Our time-lapsed analysis has further demonstrated in living embryos the role of dynein in the initial migration of centrosomes along the nuclear envelope before spindle assembly, as well as in the attachment of centrosomes to spindle poles. The inappropriate behaviors of centrosomes that result from the reduction in dynein function disrupt spindle morphogenesis. Our results show that dynein function in centrosome attachments is essential for autonomous spindle function, and the global spatial organization of early development in the Drosophila embryo.
Acknowledgments
We would like to acknowledge many fruitful discussions with members of the laboratory and thank the Imaging Center staff for help with the confocal instrumentation and image analysis.
This work has been supported by grants from the National Institutes of Health (NIH), the PEW Foundation, and the American Heart Foundation (to T.S. Hays). NIH fellowships provided support for E. Wojcik and J. Robinson.
Footnotes
1.used in this paper: Dhc, dynein heavy chain; NEBD, nuclear envelope breakdown
J. Robinson and E. Wojcik contributed equally to this work.
References
- Allan V. Organelle movement. Dynactinportrait of a dynein regulator. Curr. Biol. 1994;4:1000–1002. doi: 10.1016/s0960-9822(00)00225-6. [DOI] [PubMed] [Google Scholar]
- Boleti H., Karsenti E., Vernos I. Xklp2, a novel Xenopus centrosomal kinesin-like protein required for centrosome separation during mitosis. Cell. 1996;84:49–59. doi: 10.1016/s0092-8674(00)80992-7. [DOI] [PubMed] [Google Scholar]
- Bruno K.S., Tinsley J.H., Minke P.F., Plamann M. Genetic interactions among cytoplasmic dynein, dynactin, and nuclear distribution mutants of Neurospora crassa . Proc. Natl. Acad. Sci. USA. 1996;93:4775–4780. doi: 10.1073/pnas.93.10.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busson S., Dujardin D., Moreau A., Dompierre J., De Mey J.R. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr. Biol. 1998;8:541–544. doi: 10.1016/s0960-9822(98)70208-8. [DOI] [PubMed] [Google Scholar]
- Callaini G., Dallai R., Riparbelli M.G. Cytochalasin induces spindle fusion in the syncytial blastoderm of the early Drosophila embryo. Biol. Cell. 1992;74:249–254. doi: 10.1016/0248-4900(92)90035-y. [DOI] [PubMed] [Google Scholar]
- Desai A., Deacon H.W., Walczak C.E., Mitchison T.J. A method that allows the assembly of kinetochore components onto chromosomes condensed in clarified Xenopus egg extracts. Proc. Natl. Acad. Sci. USA. 1997;94:12378–12383. doi: 10.1073/pnas.94.23.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin D., Wacker U.I., Moreau A., Schroer T.A., Rickard J.E., De Mey J.R. Evidence for a role of CLIP-170 in the establishment of metaphase chromosome alignment. J. Cell Biol. 1998;141:849–862. doi: 10.1083/jcb.141.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverri C.J., Paschal B.M., Vaughan K.T., Vallee R.B. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel D., Urrestarazu L.A., Vissers S., Jauniaux J.C., van Vliet-Reedijk J.C., Planta R.J., Gibbons I.R. Cytoplasmic dynein is required for normal nuclear segregation in yeast. Proc. Natl. Acad. Sci. USA. 1993;90:11172–11176. doi: 10.1073/pnas.90.23.11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner N.E., Vig B., Echeverri C.J., Wordeman L., Vallee R.B. Localization of motor-related proteins and associated complexes to active, but not inactive, centromeres. Hum. Mol. Genet. 1998;7:671–677. doi: 10.1093/hmg/7.4.671. [DOI] [PubMed] [Google Scholar]
- Foe V.E., Alberts B.M. Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J. Cell Sci. 1983;61:31–70. doi: 10.1242/jcs.61.1.31. [DOI] [PubMed] [Google Scholar]
- Gaglio T., Dionne M.A., Compton D.A. Mitotic spindle poles are organized by structural and motor proteins in addition to centrosomes. J. Cell Biol. 1997;138:1055–1066. doi: 10.1083/jcb.138.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepner J., Li M., Ludmann S., Kortas C., Boylan K., Iyadurai S.J., McGrail M., Hays T.S. Cytoplasmic dynein function is essential in Drosophila melanogaster . Genetics. 1996;142:865–878. doi: 10.1093/genetics/142.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I.R. Dynein family of motor proteinspresent status and future questions. Cell Motil. Cytoskel. 1995;32:136–144. doi: 10.1002/cm.970320214. [DOI] [PubMed] [Google Scholar]
- Gonzalez C., Glover D.M. Techniques for studying mitosis in Drosophila . In: Fantes P., Brooks R., editors. The Cell CycleA Practical Approach. IRL Press at Oxford University Press; Oxford, United Kingdom: 1994. pp. 143–175. [Google Scholar]
- Hays T.S., Porter M.E., McGrail M., Grissom P., Gosch P., Fuller M.T., McIntosh J.R. A cytoplasmic dynein motor in Drosophilaidentification and localization during embryogenesis. J. Cell Sci. 1994;107:1557–1569. doi: 10.1242/jcs.107.6.1557. [DOI] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Blank T., Sandaltzopoulos R., Becker P., Hyman A., Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Habermann A., Karsenti E., Hyman A. Spindle assembly in Xenopus egg extractsrespective roles of centrosomes and microtubule self-organization. J. Cell Biol. 1997;138:615–628. doi: 10.1083/jcb.138.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran E.A., Karki S., Holzbaur E.L. The role of the dynactin complex in intracellular motility. Int. Rev. Cytol. 1998;182:69–109. doi: 10.1016/s0074-7696(08)62168-3. [DOI] [PubMed] [Google Scholar]
- Holzbaur E.L.F., Vallee R.B. Dyneinsmolecular structure and cellular function. Annu. Rev. Cell Biol. 1994;10:339–372. doi: 10.1146/annurev.cb.10.110194.002011. [DOI] [PubMed] [Google Scholar]
- Hyman A.A. Preparation of marked microtubules for the assay of the polarity of microtubule-based motors by fluorescence. J. Cell Sci. Suppl. 1991;14:125–127. doi: 10.1242/jcs.1991.supplement_14.25. [DOI] [PubMed] [Google Scholar]
- Hyman A.A., Karsenti E. Morphogenetic properties of microtubules and mitotic spindle assembly. Cell. 1996;84:401–410. doi: 10.1016/s0092-8674(00)81285-4. [DOI] [PubMed] [Google Scholar]
- Inoue S., Turgeon B.G., Yoder O.C., Aist J.R. Role of fungal dynein in hyphal growth, microtubule organization, spindle pole body motility and nuclear migration. J. Cell Sci. 1998;111:1555–1566. doi: 10.1242/jcs.111.11.1555. [DOI] [PubMed] [Google Scholar]
- Karki S., LaMonte B., Holzbaur E.L. Characterization of the p22 subunit of dynactin reveals the localization of cytoplasmic dynein and dynactin to the midbody of dividing cells [published erratum appears in J. Cell Biol. 1998. 143:561] J. Cell Biol. 1998;142:1023–1034. doi: 10.1083/jcb.142.4.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenti E. Mitotic spindle morphogenesis in animal cells. Semin. Cell Biol. 1991;2:251–260. [PubMed] [Google Scholar]
- Kashina A.S., Rogers G.C., Scholey J.M. The bimC family of kinesinsessential bipolar mitotic motors driving centrosome separation. Biochim. Biophys. Acta. 1997;1357:257–271. doi: 10.1016/s0167-4889(97)00037-2. [DOI] [PubMed] [Google Scholar]
- Keating T.J., Peloquin J.G., Rodionov V.I., Momcilovic D., Borisy G.G. Microtubule release from the centrosome. Proc. Natl. Acad. Sci. USA. 1997;94:5078–5083. doi: 10.1073/pnas.94.10.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Wisniewski J.C., Dentler W.L., Asai D.J. Gene knockouts reveal separate functions for two cytoplasmic dyneins in Tetrahymena thermophila . Mol. Biol Cell. 1999;10:771–784. doi: 10.1091/mbc.10.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.G., McGrail M., Serr M., Gepner J., Hays T.S. Drosophila cytoplasmic dynein, a microtubule motor that is asymmetrically localized in the oocyte. J. Cell Biol. 1994;126:1475–1494. doi: 10.1083/jcb.126.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.Y., Yeh E., Hays T., Bloom K. Disruption of mitotic spindle orientation in a yeast dynein mutant. Proc. Natl. Acad. Sci. USA. 1993;90:10096–10100. doi: 10.1073/pnas.90.21.10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrail M., Hays T.S. The microtubule motor cytoplasmic dynein is required for spindle orientation during germline cell divisions and oocyte differentiation in Drosophila . Development. 1997;124:2409–2419. doi: 10.1242/dev.124.12.2409. [DOI] [PubMed] [Google Scholar]
- McGrail M., Gepner J., Silvanovich A., Ludmann S., Serr M., Hays T.S. Regulation of cytoplasmic dynein function in vivo by the Drosophila Glued complex. J. Cell Biol. 1995;131:411–425. doi: 10.1083/jcb.131.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A., Cleveland D.W. Pathways of spindle pole formationdifferent mechanisms; conserved components. J. Cell Biol. 1997;138:953–956. doi: 10.1083/jcb.138.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A., Ramyar K., Vechio J.D., Cleveland D.W. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell. 1996;87:447–458. doi: 10.1016/s0092-8674(00)81365-3. [DOI] [PubMed] [Google Scholar]
- Micklem D.R., Dasgupta R., Elliott H., Gergely F., Davidson C., Brand A., Gonzalez-Reyes A., St. Johnston D. The mago nashi gene is required for the polarisation of the oocyte and the formation of perpendicular axes in Drosophila . Curr. Biol. 1997;7:468–478. doi: 10.1016/s0960-9822(06)00218-1. [DOI] [PubMed] [Google Scholar]
- Minden J.S., Agard D.A., Sedat J.W., Alberts B.M. Direct cell lineage analysis in Drosophila melanogaster by time-lapse, three-dimensional optical microscopy of living embryos. J. Cell Biol. 1989;109:505–516. doi: 10.1083/jcb.109.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr C.M., Coue M., Grissom P.M., Hays T.S., Porter M.E., McIntosh J.R. Cytoplasmic dynein is localized to kinetochores during mitosis. Nature. 1990;345:263–265. doi: 10.1038/345263a0. [DOI] [PubMed] [Google Scholar]
- Plamann M., Minke P.F., Tinsley J.H., Bruno K.S. Cytoplasmic dynein and actin-related protein Arp1 are required for normal nuclear distribution in filamentous fungi. J. Cell Biol. 1994;127:139–149. doi: 10.1083/jcb.127.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postner M.A., Miller K.G., Wieschaus E.F. Maternal effect mutations of the sponge locus affect actin cytoskeletal rearrangements in Drosophila melanogaster embryos. J. Cell Biol. 1992;119:1205–1218. doi: 10.1083/jcb.119.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinsch S., Gonczy P. Mechanisms of nuclear positioning. J. Cell Sci. 1998;111:2283–2295. doi: 10.1242/jcs.111.16.2283. [DOI] [PubMed] [Google Scholar]
- Saunders W.S., Koshland D., Eshel D., Gibbons I.R., Hoyt M.A. Saccharomyces cerevisiae kinesin- and dynein-related proteins required for anaphase chromosome segregation. J. Cell Biol. 1995;128:617–624. doi: 10.1083/jcb.128.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D.J., McDonald K.L., Brown H.M., Matthies H.J., Walczak C., Vale R.D., Mitchison T.J., Scholey J.M. The bipolar kinesin, KLP61F, cross-links microtubules within interpolar microtubule bundles of Drosophila embryonic mitotic spindles. J. Cell Biol. 1999;144:125–138. doi: 10.1083/jcb.144.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr D.A., Williams B.C., Hays T.S., Goldberg M.L. ZW10 helps recruit dynactin and dynein to the kinetochore. J. Cell Biol. 1998;142:763–774. doi: 10.1083/jcb.142.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer E.R., Wordeman L., Schroer T.A., Sheetz M.P. Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature. 1990;345:266–268. doi: 10.1038/345266a0. [DOI] [PubMed] [Google Scholar]
- Sullivan W., Minden J.S., Alberts B.M. Daughterless-abo-like, a Drosophila maternal-effect mutation that exhibits abnormal centrosome separation during the late blastoderm divisions. Development. 1990;110:311–323. doi: 10.1242/dev.110.2.311. [DOI] [PubMed] [Google Scholar]
- Vaisberg E.A., Koonce M.P., McIntosh J.R. Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J. Cell Biol. 1993;123:849–858. doi: 10.1083/jcb.123.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes-Perez R.E., Minden J.S. Drosophila melanogaster syncytial nuclear divisions are patternedtime-lapse images, hypothesis and computational evidence. J. Theor. Biol. 1995;175:525–532. doi: 10.1006/jtbi.1995.0160. [DOI] [PubMed] [Google Scholar]
- Vallee R.B., Vaughan K.T., Echeverri C.J. Targeting of cytoplasmic dynein to membranous organelles and kinetochores via dynactin. Cold Spring Harb. Symp. Quant. Biol. 1995;60:803–811. doi: 10.1101/sqb.1995.060.01.086. [DOI] [PubMed] [Google Scholar]
- Verde F., Berrez J.M., Antony C., Karsenti E. Taxol-induced microtubule asters in mitotic extracts of Xenopus eggsrequirement for phosphorylated factors and cytoplasmic dynein. J. Cell Biol. 1991;112:1177–1187. doi: 10.1083/jcb.112.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak C.E., Vernos I., Mitchison T.J., Karsenti E., Heald R. A model for the proposed roles of different microtubule-based motor proteins in establishing spindle bipolarity. Curr. Biol. 1998;8:903–913. doi: 10.1016/s0960-9822(07)00370-3. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer C.M., Karki S.B., Kuznetsov S.A., Tabb J.S., Weiss D.G., Langford G.M., Holzbaur E.L. The interaction between cytoplasmic dynein and dynactin is required for fast axonal transport. Proc. Natl. Acad. Sci. USA. 1997;94:12180–12185. doi: 10.1073/pnas.94.22.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann T., Boleti H., Antony C., Karsenti E., Vernos I. Localization of the kinesin-like protein Xklp2 to spindle poles requires a leucine zipper, a microtubule-associated protein, and dynein. J. Cell Biol. 1998;143:673–685. doi: 10.1083/jcb.143.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordeman L., Mitchison T.J. Identification and partial characterization of mitotic centromere-associated kinesin, a kinesin-related protein that associates with centromeres during mitosis. J. Cell Biol. 1995;128:95–104. doi: 10.1083/jcb.128.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X., Beckwith S.M., Morris N.R. Cytoplasmic dynein is involved in nuclear migration in Aspergillus nidulans . Proc. Natl. Acad. Sci. USA. 1994;91:2100–2104. doi: 10.1073/pnas.91.6.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X., Osmani A.H., Osmani S.A., Roghi C.H., Willins D.A., Beckwith S., Goldman G., Chiu Y., Xin M., Liu B. Analysis of nuclear migration in Aspergillus nidulans . Cold Spring Harb. Symp. Quant. Biol. 1995;60:813–819. doi: 10.1101/sqb.1995.060.01.087. [DOI] [PubMed] [Google Scholar]