Abstract
γ-Tubulin is a centrosomal component involved in microtubule nucleation. To determine how this molecule behaves during the cell cycle, we have established several vertebrate somatic cell lines that constitutively express a γ-tubulin/green fluorescent protein fusion protein. Near simultaneous fluorescence and DIC light microscopy reveals that the amount of γ-tubulin associated with the centrosome remains relatively constant throughout interphase, suddenly increases during prophase, and then decreases to interphase levels as the cell exits mitosis. This mitosis-specific recruitment of γ-tubulin does not require microtubules. Fluorescence recovery after photobleaching (FRAP) studies reveal that the centrosome possesses two populations of γ-tubulin: one that turns over rapidly and another that is more tightly bound. The dynamic exchange of centrosome-associated γ-tubulin occurs throughout the cell cycle, including mitosis, and it does not require microtubules. These data are the first to characterize the dynamics of centrosome-associated γ-tubulin in vertebrate cells in vivo and to demonstrate the microtubule-independent nature of these dynamics. They reveal that the additional γ-tubulin required for spindle formation does not accumulate progressively at the centrosome during interphase. Rather, at the onset of mitosis, the centrosome suddenly gains the ability to bind greater than three times the amount of γ-tubulin than during interphase.
Keywords: centrosome, mitosis, γ-tubulin, green fluorescent protein, microtubules
All animal cells possess an organelle, known as a centrosome (or spindle pole body), that plays a major role in establishing the microtubule (Mt)1 cytoskeleton. In vertebrates, this organelle consists of a mother and daughter centriole pair (i.e., a diplosome) that is surrounded by an ill-defined matrix known as the pericentriolar material. It is the pericentriolar material that contains the proteins responsible for Mt assembly (Gould and Borisy 1977). We now know that Mt nucleation within the pericentriolar material occurs in association with a special tubulin isoform (γ-tubulin; Oakley and Oakley 1989) which, along with several other proteins, forms 25-nm-diam ring-like complexes (γTuRCs) that nucleate Mt assembly (Moritz et al. 1995; Zheng et al. 1995).
The centrosome undergoes complex and orderly changes as the cell progresses through interphase into mitosis. In actively cycling cells, the centrioles replicate precisely once per cell cycle near the time of DNA synthesis (reviewed in Hinchcliffe et al. 1999; Lacey et al. 1999). However, at a functional level, the ability of the centrosome to generate Mts appears to remain relatively constant throughout interphase, but then dramatically increases as the cell enters mitosis (Snyder and McIntosh 1975; Brinkley et al. 1981; Kuriyama and Borisy 1981). The mechanism by which the centrosome suddenly becomes activated at the G2/M transition, which is also known as the maturation process, remains largely unknown (reviewed in Kellogg et al. 1994; Sluder and Rieder 1996; Paoletti and Bornens 1997).
Mazia 1987 envisioned that the centrosome is composed of a linear structural element to which the functional units for Mt nucleation are attached. In his model, the Mt-nucleating potential of the centrosome is regulated by folding and unfolding of this structural element which, in turn, exposes different numbers of Mt nucleating sites. Recent studies confirm that the centrosome is composed of an insoluble structural backbone, termed the centromatrix, which is organized around a uniform fibril (Schnackenberg et al. 1998). Furthermore, the Mt-nucleating potential of the centromatrix can be restored by simply adding back functional components, including γTuRCs (Moritz et al. 1998; Schnackenberg et al. 1998). Although the molecular definition of the centromatrix remains to be determined, at least one structural protein has been identified that forms a lattice-like meshwork around the centrioles in vertebrates. This protein, known as pericentrin (Doxsey et al. 1994), also forms a complex with γ-tubulin (Dictenberg et al. 1998).
As the cell enters mitosis, its centrosomes become heavily phosphorylated (reviewed in Centonze and Borisy 1990; Glover et al. 1996; Nigg 1998) and this chemical change correlates temporally with a sudden increase in the Mt-nucleating potential of the centrosome. It is currently unclear how this phosphorylation leads to enhanced Mt nucleation. An important question related to this topic is: when are the components required for enhanced Mt assembly (e.g., γTuRCs) recruited to the centrosome? One possibility is that the centrosome grows gradually during the cell cycle, recruiting both structural and functional elements that are then maintained in an inactive form until needed (e.g., Rieder and Borisy 1982; Dictenberg et al. 1998). Another possibility is that the biochemical changes induced by phosphorylation near the onset of mitosis lead to the sudden recruitment of Mt nucleating elements (e.g., Zheng et al. 1991; Lajoie-Mazenc et al. 1994).
It is well established that zygotes contain a large pool of inactive γ-tubulin that is not directly involved in Mt assembly. At each cell cycle, some of the γ-tubulin in this cytoplasmic reservoir is used to form the additional centrosomes required for early development (Gard et al. 1990; Felix et al. 1994). Recent biochemical studies have shown that, even in vertebrate somatic cells, 80% of the γ-tubulin is present in the cytoplasm and is not bound to the centrosome (Moudjou et al. 1996). An important unresolved issue is whether this cytoplasmic pool simply represents a store for future demands as in zygotes, or if it is used throughout the cell cycle. In this respect, elegant video–light microscopy (LM) studies reveal that Mts constantly detach and move away from their site of nucleation within the interphase centrosome (Keating et al. 1997). One idea is that the minus ends of these Mts are capped by γ-tubulin. However, if this is true, then the centrosome would gradually become depleted of its Mt-nucleating potential unless a mechanism exists to replenish its γ-tubulin supply. Thus, the questions of whether centrosome-associated γ-tubulin is in dynamic exchange with a cytoplasmic pool and whether this exchange depends on Mt dynamics are important to understanding centrosome function.
Until recently, these and related questions could only be addressed indirectly by analyzing data obtained in vitro or from fixed cell preparations. Although these analyses identified a variety of bona fide centrosomal components and provided a general outline of cell-cycle related changes in centrosome composition, none were designed to follow the real-time behavior of an individual centrosomal component in vivo. As a result, we know very little about the dynamic properties of the major centrosomal proteins, including γ-tubulin. To overcome the limitations inherent in fixed-cell studies, we have established several permanent vertebrate cells lines that constitutively express a low level of green fluorescent protein (GFP)-tagged γ-tubulin. This protein accumulates in and delineates the boundary of the centrosome throughout the cell cycle without inducing any detectable aberrations. The ability to quantify the centrosome-associated GFP-fluorescence and to follow fluorescence recovery after photobleaching (FRAP) has enabled us to explore how this protein behaves during the cell cycle. The results of these studies reveal that the centrosome in vertebrate somatic cells possesses two populations of γ-tubulin: one that is in a rapid exchange with the cytoplasmic pool and another that exchanges slowly. Our data also reveal for the first time that the activation of the centrosome at the beginning of mitosis corresponds with its sudden recruitment of γ-tubulin. Importantly, the dynamic behavior of centrosome-associated γ-tubulin does not depend on the presence of Mts. Finally, our in vivo observations on γ-tubulin distribution during the cell cycle confirm previous reports that the localization of this protein is not limited to the centrosome proper, but that it also accumulates within the mitotic spindle during metaphase and sometimes in the midbody during cytokinesis.
Materials and Methods
Construction of a γTGFP Plasmid
To construct a γ-tubulin/green fluorescence protein (γTGFP) expressing plasmid we started with a full-length human γ-tubulin sequence (Zheng et al. 1991). The original stop codon was destroyed and a 5′-terminal BamHI and a 3′-terminal HindIII site were introduced by PCR. The resultant fragment was then cloned into pcDNA3 vector (CLONTECH Laboratories, Inc.). The S65T variant of GFP (CLONTECH Laboratories, Inc.) was fused to the γ-tubulin COOH terminus. This resulting plasmid was designated as pcDNA3–γTGFP.
Cell Cultures
PtK1 (rat kangaroo kidney epithelial) cells were purchased from American Type Culture Collection at passage 69 and grown in antibiotic-free Ham's F12 media supplemented with 10% FCS. At passage 80, cells were transformed with the pcDNA3–γTGFP plasmid by electroporation. γTGFP-expressing clones were initially isolated by G418-resistance selection (1 mg/ml). Of these clones, several were then selected for the lowest expression level that still yielded a sufficient GFP-fluorescence signal for time-lapse microscopy with a Photometrics PXL cooled CCD camera. This strategy enabled us to avoid potential abnormal phenotypes due to γ-tubulin overexpression (e.g., Shu and Joshi 1995). Three selected clones (PtKG-22, PtKG-23, and PtKG-36) were finally purified by limited-dilution cloning on a feeder layer of wild-type PtK1 cells. All three of these clones appear to be stable and continue to express γTGFP (as judged by centrosome-associated GFP signal) after >20 passages in the absence of G418. All three clones behaved identically in the experiments described in this paper. A similar strategy was used to isolate clones constitutively expressing γTGFP from PK (pig kidney epithelial) and CV-1 (green monkey kidney fibroblasts).
All experiments were conducted on cells grown on #1 1/2 coverslips mounted in Rose chambers in L-15 media, as previously described (Rieder et al. 1994, Rieder et al. 1995). Cells were kept at 37°C using a custom built microscope stage heater (Rieder and Cole 1998a). For experiments involving Mt disassembly, Rose chamber cultures were treated with 4 μM nocodazole 1 h before observations.
Time-lapse GFP/DIC Imaging and Intensity Measurements
Near simultaneous GFP fluorescence/DIC time-lapse sequences were collected using a custom-modified Nikon Optiphot microscope equipped with De Senarmont compensation long-working-distance DIC optics (60× 1.4 NA PlanApo lens), a Quad-Fluor epifluorescence attachment (Nikon, Inc.), a stepping motor for Z-positioning (Ludl Electronics), and a Photometrics PXL cooled CCD camera (Photometric).
The microscope system was driven by Isee software (Inovision Corp.), and images were recorded as 12-bit computer files (0–4095 pixel intensity). The intensity of brightest pixels in the fluorescence images were kept at <600, which guaranteed that none of the images were saturated. The CCD chip was read out at 800 kHz with an electronic gain of four, which assured a linear correspondence between the well-charge and light intensity for the PXL camera.
To capture the full in-focus intensity for centrosomes that move in all three axes (X, Y, and Z) within a living cell, the GFP image for any one time point was collected as Z-series of 16 images at 0.5-μm steps. From these Z-series, a single maximal intensity projection was computed for each individual time point. These computations were done concurrent with image collection and only the resultant maximal intensity projections were saved to the disk and subsequently used for image analysis. The DIC images were acquired at the focal plane corresponding to the middle of the GFP Z-sequence.
All images were corrected using standard algorithm (e.g., Zhai and Borisy 1994), as follows:
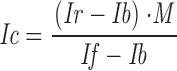 |
1 |
where Ic is the corrected image, Ir is the noncorrected or raw object exposure, Ib is an electronic or dark background frame obtained with the shutter closed, M is the mean pixel value of the object exposure, and If is flat field image obtained with no specimen, but a homogeneous fluorescent field.
To measure the amount of γTGFP associated with the centrosome, a circle of 20 pixels in diameter (Ø1.75 μm) was centered on the centrosome and the sum of pixel intensities was calculated. The results of these intensity measurements were normalized so that the highest value for the centrosome was equal to ten while the background intensity outside of the cell was zero.
For fluorescence imaging, cells were illuminated with light from a 75 W xenon burner that was filtered with a GG400 (to eliminate UV), a KG5 (to eliminate IR), and a 4× or 8× ND filter to decrease light intensity to a level safe for the cells. The DM505 filter cube (450–490 nm excitation − 520–560 nm emission; Nikon, Inc.) was used for GFP detection. For DIC imaging, cells were illuminated with light from a 50 W tungsten filament, filtered with GG400, KG5, and GIF546 (green) filters.
Both the fluorescence and DIC light sources were shuttered by UniBlitz shutters (Uniblitz Electonics) so that cells were illuminated only during image acquisition (200 ms/frame for GFP and 600 ms/frame for DIC mode). Under these conditions, we were able to follow centrosomes in interphase cells for more than six hours at a framing rate of one fluorescence sequence (i.e., 16 frames × 200 ms = 3.2 s total illumination) every 2.5 min without detectable photobleaching.
We find, as reported by others (e.g., Manders et al. 1999), that vertebrate somatic cells are extremely sensitive to illumination, even with monochromatic light, and that overillumination can forestall progression through the cell cycle and even send cells in prophase back to interphase (see Results).
Photobleaching of the Centrosome-associated γTGFP
For photobleaching experiments, we used a continuous-wave argon ion laser (model 2010; Uniphase Corp.). The output light was filtered by a laser-quality 488-nm interference filter and extended using a 10× beam-extender. The 12-mm-diam beam was directed to the back aperture of the lens through a custom-made additional epiport. The objective lens then focused the beam into a small spot (∼1.5 μm) within the specimen plane. This approach allows one to photobleach individual objects 1–2 μm in diam with minimal light exposure to the surroundings. In our experiments, we were able to photobleach one of two replicated centrosomes that were separated by ∼5 μm with no detectable decrease in the signal intensity associated with the other centrosome.
For photobleaching, the light intensity was empirically adjusted so that an ∼5–10 s exposure completely abolished the GFP signal associated with the centrosome. Under this condition, the centrosomes always recovered after photobleaching and the cells eventually entered mitosis and formed a normal spindle (see Results).
Immunostaining
For immunostaining, cells were briefly rinsed in warm (∼37°C) PEM buffer (100 mM Pipes, pH 6.9, 5 mM EGTA, 1 mM MgCl2), permeabilized for 30 s in PEM with 0.1% Triton X-100, and fixed in 1% glutaraldehyde in PEM. After fixation, free aldehyde groups were reduced by a 5-min incubation in NaBH4 (1 mg/ml). γ-Tubulin was stained using a mouse mAb (clone GTU-88, Sigma Chemical Co.) and a TRITC-conjugated goat anti–mouse IgG secondary antibody (Sigma Chemical Co.). α-Tubulin was stained using a rat mAb (clone YL1/2; kind gift of Dr. J.V. Kilmartin, MRC, Cambridge, UK) and an FITC-conjugated donkey anti–rat IgG secondary antibody (Jackson ImmunoResearch Laboratories, Inc.).
Since two separated centrosomes are often located in different focal planes, all fluorescence images were collected as Z-series (200-nm steps) on the same microscope workstation used for GFP-imaging. These datasets were then deconvolved using Delta Vision deconvolution software (Applied Precision Inc.) and presented as maximal intensity projection.
Results
We have established several stable cell lines that constitutively express the γTGFP. Among them are clones isolated from two different epithelial cell lines, including PtKG (from the parental PtK1, rat kangaroo kidney) and PKG (from the parental PK, pig kidney), as well as a CVG fibroblastic cell line (from parental CV-1, green monkey kidney). In this report, we illustrate our findings primarily with video sequences obtained from PtKG cells. However, without exception, the same results were obtained from CVG and PKG cells.
The constitutive expression of low γTGFP levels is not toxic to the cells. All of our γTGFP expressing lines exhibited growth rates similar to the parental cell lines. The mitotic index and percent of multinucleated cells and abnormal (multipolar) spindles all appeared similar to those of the parental cell lines.
When expressed in mammalian cells, γTGFP associates with the centrosome and fluorescently labels this organelle throughout the cell cycle. In interphase cells, the γTGFP-labeled centrosome appeared as two fluorescent dots that varied widely in their separation (Fig. 1). Interphase cells with widely separated centrosomes were common in PKG and CVG cells, but less abundant in PtKG cells. Previous correlative LM/EM observations (Khodjakov et al. 1997) have shown that each of these dots contains either a single centriole (G1) or a pair of centrioles (G2). For any one interphase cell, the γTGFP fluorescence intensity of each of the two dots was usually fairly similar. However, in some cells, they differed substantially and one of the dots could contain up to twice as much γTGFP as the other. In time-lapse sequences, these dots were motile, often seen to separate and then reform a common complex, only to separate again several times during the period of observation.
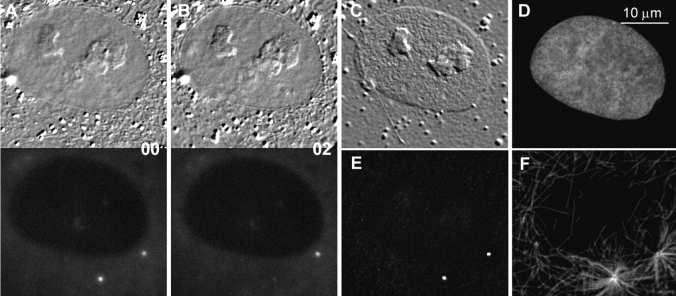
Figure 1.
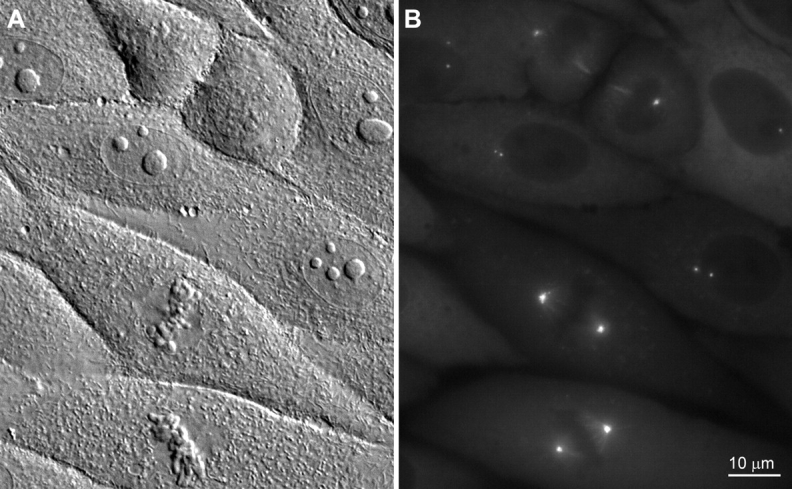
DIC (A) and corresponding epifluorescence (B) micrographs of living PKG cells. Note that the centrosomes in interphase cells consist of two dots that are either adjacent or well separated from one another.
The γTGFP fusion protein was excluded from the nucleus, but was present in significant quantities in the cytoplasm of all cells (Fig. 1). This observation is consistent with the biochemical analyses of Moudjou et al. 1996, which revealed that only 20% of the γ-tubulin in cells is associated with the centrosome, whereas ∼80% remains in the cytoplasm.
The Amount of the Centrosome-associated γTGFP Increases Dramatically as the Cell Progresses through Prophase, and then Decreases during Anaphase/Telophase
We followed interphase cells by quantitative time-lapse imaging for up to 20 h (n = 20). In all cases, the intensity of the γTGFP signal associated with the centrosome remained roughly constant throughout the observation period (data not shown).
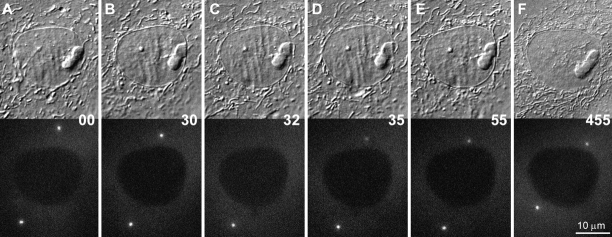
As cells entered mitosis, as defined by the initiation of chromosome condensation, the intensity of the γTGFP signal did not change significantly (Fig. 2). However, 20–30 min before nuclear envelope breakdown the γTGFP signal associated with the centrosome suddenly began to increase (Fig. 2 and Fig. 3). It then reached its maximum level, greater than three times that seen during early prophase, shortly after nuclear envelope breakdown, which can be clearly defined in these cells as the point when γTGFP entered the previously nonfluorescent nuclear volume (Fig. 2C and Fig. D).
Figure 2.
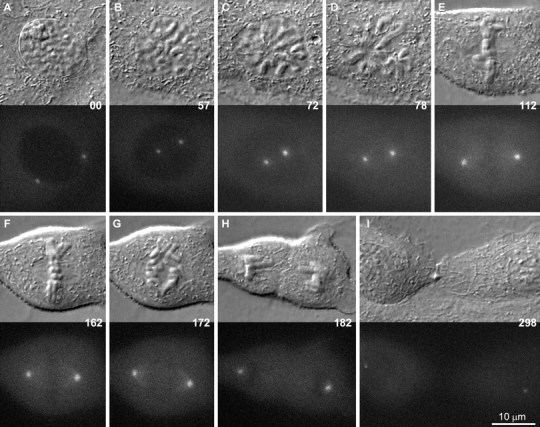
A–I, Selected frames from a time-lapse sequence of a PtKG cell progressing through mitosis. The top half of each frame presents the DIC image of the cell, while the bottom half represents the corresponding epifluorescence image. In this cell, the two centrosomes were already well separated in early prophase (A), and their γTGFP intensity remained relatively constant during the next hour (B). The γTGFP content then increased rapidly during spindle formation (C and D) and decreased as the cell exited mitosis (G–I). Time is in minutes.
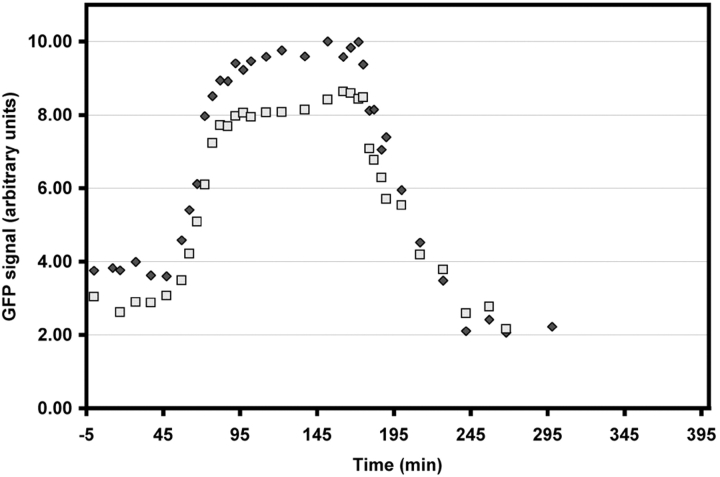
Figure 3.
Centrosome-associated γTGFP intensity versus time in the cell shown in Fig. 2. Note that the two centrosomes displayed roughly the same intensity, which remained relatively constant. At the 55 min time point, the γTGFP content of both centrosomes suddenly began to increase, and reached a maximum ∼30 min later. It then remained at this level for ∼100 min, after which time the cell entered anaphase and the γTGFP intensity dropped. Note that the residual γTGFP associated with the early G1 centrosome (time = 295 min) was ∼50% that associated with the same centrosome before its mitotic activation.
In vertebrates, the duration of spindle formation, as defined by the interval between nuclear envelope breakdown and anaphase onset, is highly variable and depends on how long the cell contains monooriented chromosomes (Rieder et al. 1994, Rieder et al. 1995). In cells in which spindle formation was prolonged, the γTGFP signal remained at its peak level until anaphase onset, which took one or more hours (Fig. 2 and Fig. 3). As soon as the cell initiated anaphase, the γTGFP content of both centrosomes progressively decreased until it reached a minimal level after cytokinesis (Fig. 2 and Fig. 3). At this point in G1, the γTGFP content of the centrosome was ∼50% of what it was during the previous G2, before its mitotic activation (see Fig. 2 and Fig. 3). Thus, from early G1 until late G2, the γTGFP content of the centrosome increased only about two times, in contrast to the sudden greater than three times increase during prophase.
In addition to an increased intensity of γTGFP, the apparent diameter of the centrosome also increased during spindle formation (Fig. 4). Shortly after nuclear envelope breakdown, and after the central part of the centrosome reached its maximal intensity, the γTGFP signal continued to progressively accumulate around the centrosomal periphery. As a result, the area occupied by the centrosome in metaphase, as defined by a γTGFP intensity similar to that of an interphase centrosome, was much larger than that seen at nuclear envelope breakdown. Here it is noteworthy that our measurements of the γTGFP amount associated with the centrosome, as presented in Fig. 3, only account for the γTGFP signal contained within the central part of the centrosome (defined by a 1.75-μm-diam circle) and, therefore, underestimates the total amount of γTGFP recruited during mitosis. We chose to use the same-size cursor for both interphase and mitotic centrosomes because it was impossible to define the exact boundary of the centrosome during the later stages of spindle formation/maturation.
Figure 4.
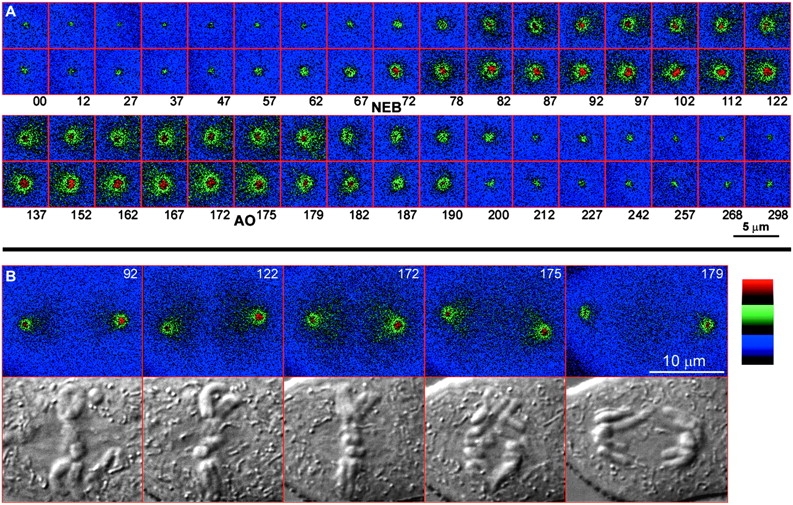
A pseudocolor map of centrosome-associated γTGFP intensity in the cell pictured in Fig. 2 and Fig. 3. A, Depicts the two centrosomes, highly magnified, as they proceed from early prophase (00 min) to the next G1 (298 min). Note that the γTGFP content of each centrosome suddenly increased in late prophase, and even after reaching its maxim intensity in its center (82) it continued to acquire additional γTGFP around its periphery. As a result, the apparent size of the centrosome during the later stages of spindle formation (122–172 min) was several times larger than in early prophase. B, Depicts the incorporation and redistribution of γTGFP within the spindle as it matured (92–172 min), and its rapid decrease after anaphase onset (172–179 min). Note that γTGFP became concentrated in each half-spindle only after the centrosome had reached its full intensity, which occurred during true metaphase (122–172 min).
As the spindle matured, the γTGFP signal extended from each centrosome into its associated half-spindle so that at anaphase onset both half-spindles also contained a γTGFP intensity equivalent to that of an interphase centrosome (Fig. 4 B). After the chromatids disjoined the γTGFP, signal in the spindle rapidly decreased to background levels by late anaphase (172 and 179 time points in Fig. 4 B).
In some cells, we observed an accumulation of the γTGFP signal at the ends of midbody Mts during cytokinesis (e.g., see Fig. 1). This phenomenon was regularly seen in PKG cells, but only rarely in PtKG and CVG cells.
The Recruitment of γTGFP to the Centrosome during Mitosis Does Not Require Microtubules
Is the sudden increase of centrosome-associated γTGFP during prophase mediated by Mts? To answer this question, we followed cells by time-lapse microscopy (n = 10) as they entered mitosis under conditions in which they lacked Mts (4 μM nocodazole for 1 h). As expected, this treatment inhibited centrosome movement and spindle formation (Fig. 5). However, it did not inhibit the sudden accumulation of γTGFP at the centrosome as the cell progressed through prophase (Fig. 6). This accumulation occurred with kinetics similar to those seen in untreated cells (Fig. 3 and Fig. 6), and the γTGFP level remained maximal for as long as the cell was blocked in mitosis (Fig. 6). Thus, the recruitment of γTGFP to the centrosome during mitosis does not depend on the presence of Mts.
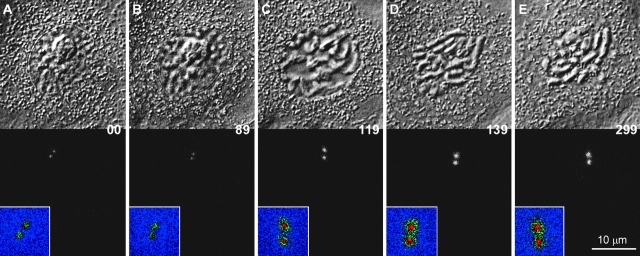
Figure 5.
The sudden incorporation of γTGFP into the centrosome during the early stages of mitosis does not depend on the presence of Mts. In this example, a PtKG cell entered mitosis (A–C) in the presence of 4 μM nocodazole. Note that the γTGFP intensity of both centrosomes remained relatively constant between A and B, and then suddenly increased in late prophase (C) and remained high during the mitotic arrest (D–E). The pseudocolored inserts in each frame use the same color map as in Fig. 4, and the centrosomes are magnified two times from the black and white images.
Figure 6.
Centrosome-associated γTGFP intensity versus time in the cell shown in Fig. 5. A comparison of this plot from a nocodazole treated cell, with that of control cells containing Mts (Fig. 3), reveals that the γTGFP content of the centrosome increased with approximately the same kinetics during early mitosis, regardless of whether Mts were present.
Unlike mitosis in control cells, the γTGFP signal in nocodazole treated cells remained closely associated with the centrosome, and, after reaching a maximal level, it did not continue to accumulate around the centrosomal periphery (Fig. 4 A and 5).
The γTGFP Content of Prophase Centrosomes, Induced to Return to G2 by Excessive Illumination, Decreases to Interphase Levels
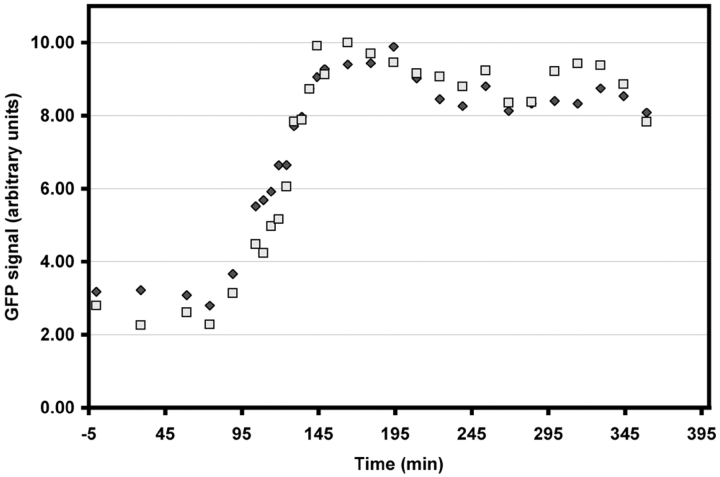
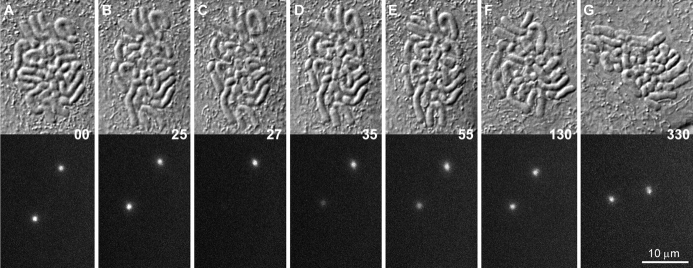
When vertebrate cells in prophase are excessively irradiated, they decondense their chromosomes and return to G2. In PtK1 cells, this reversal of the cell cycle is correlated with the dephosphorylation of those epitopes phosphorylated during the nuclear events of prophase (Rieder and Cole 1998b). In some instances, the prophase cells that we were following by time-lapse LM decondensed their chromosomes and returned to interphase (n = 4), which we assume was due to excessive illumination (see also Rieder and Cole 1998b; Manders et al. 1999). Under this condition, we found that this reversal of the cell cycle can occur even after the centrosomes have recruited near maximal levels of γTGFP (Fig. 7). During the reversion process, the chromosomes decondensed and the amount of γTGFP associated with the centrosomes progressively decreased to typical G2 levels (Fig. 8). It then remained at this level for as long as the cell was blocked in G2.
Figure 7.
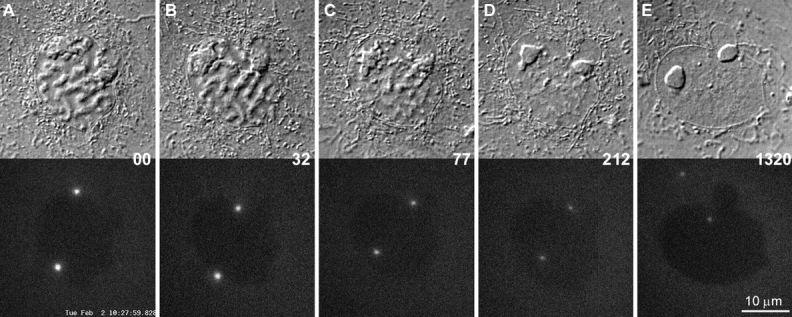
The γTGFP content of the prophase centrosome can be downregulated by inducing the cell to return to interphase. A–E are selected frames depicting a prophase PtKG as it progressively returned to G2 in response to excessive illumination (see text for details). In this example, the elevated γTGFP content of the two separated centrosomes progressively decreased as the chromosomes decondensed (A–D). It then remained constant until filming was terminated ∼18 h later (D–E).
Figure 8.
Centrosome-associated γTGFP intensity versus time in the cell shown in Fig. 7. In response to a radiation-induced reversal of the cell cycle, the γTGFP content of both separated prophase centrosome steadily decreased over a three hour period. It then remained at interphase levels for the next 18 h.
Centrosome-associated γTGFP Turns Over Constantly during Interphase and Mitosis
At this point, our data clearly demonstrated that centrosomes suddenly recruit additional γTGFP at the G2/M transition, and that this γTGFP is then lost as the cell exits mitosis. This finding raises the question as to whether centrosome-associated γTGFP is in continuous exchange with a cytoplasmic pool during interphase and mitosis.
To examine this issue, we performed FRAP studies on γTGFP-labeled centrosomes. For these studies, we chose cells with widely separated centrosomes so that we could follow fluorescence recovery of the experimental centrosome in the presence of an internal control. In all cases, when one of the centrosomes was photobleached the fluorescence intensity of the remaining centrosome remained unaffected.
To determine if our photobleaching protocol causes functional damage to the irradiated centrosome, we photobleached one of two separated centrosomes in cells treated with 4 μM nocodazole for 2 h before the experiment. This nocodazole concentration completely depolymerizes Mts. Immediately (less than one minute) after photobleaching, the cells were washed in a large volume of warm culture media for about three minutes, and then fixed and immunostained for γ-tubulin and α-tubulin (to visualize Mts). In all cases, the photobleached centrosome was found to contain a normal amount of γ-tubulin and was capable of nucleating the same number of Mt as the nonirradiated centrosome in the same cell (Fig. 9). Thus, based on functional criteria, our photobleaching protocol does not damage the centrosome.
Figure 9.
Photobleaching centrosome-associated γTGFP does not affect the Mt-nucleation potential of the centrosome. In this experiment, CVG cells were incubated in 4 μM nocodazole for two hours to disassemble the Mts. (A and B) DIC/fluorescence images of a cell before (A) and several seconds after (B) one of its two separated centrosome was photobleached by 488-nm laser light. Immediately after photobleaching, nocodazole was washed out and the cell was fixed (∼3 min after B) as new Mts were polymerizing. C–F shows the same cell after fixation and staining with Hoechst 33342 (D), anti–γ-tubulin (E), and anti–α-tubulin (F) antibodies. Note that the photobleached centrosome has a normal content of γ-tubulin (E) and that it has nucleated numbers of Mt similar to that of the nonirradiated centrosome (F).
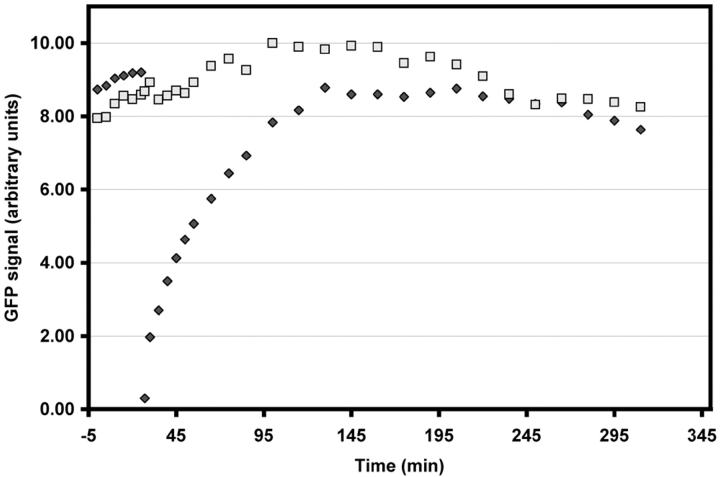
We found that when a centrosome was photobleached during interphase, it rapidly recovered ∼50% of its original signal intensity over a 60 min period (Fig. 10 and Fig. 11). It then remained at this level for several hours. In some cases (n = 7), the photobleached centrosome eventually recovered to its original intensity 5–6 h after photobleaching. However, some cells (n = 4) entered mitosis before the slow phase of recovery was completed, and when this occurred, the γTGFP content of both centrosomes increased with normal kinetics (data not shown). The same recovery curves were observed when photobleaching was performed on interphase cells treated with 4 μM nocodazole (data not shown). Together, these data reveal that two populations of γTGFP are associated with an interphase centrosome: one that turns over relatively rapidly and another that exchanges very slowly. Importantly, this dynamic exchange does not require the presence of Mts.
Figure 10.
The γTGFP associated with interphase centrosomes is in dynamic exchange with a cytoplasmic pool. A–F, Selected frames from a time-lapse recording depicting the recovery of fluorescence after photobleaching one of the centrosomes in a PtKG cell. In this example, the top centrosome was photobleached at the 32 min time point and its recovery was followed for eight hours.
Figure 11.
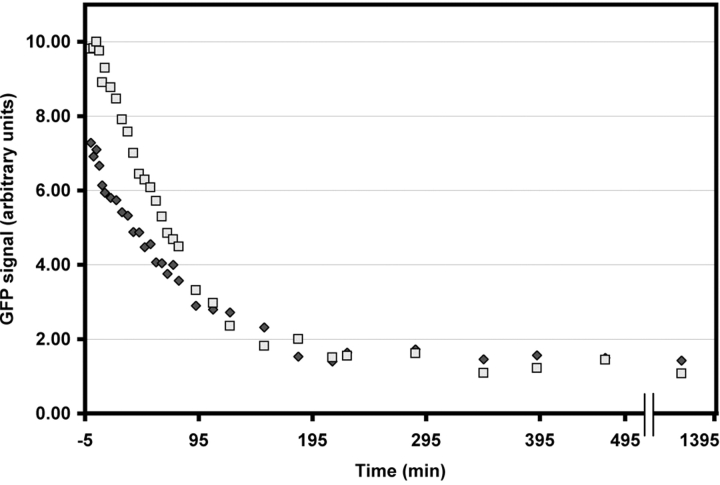
Centrosome-associated γTGFP intensity versus time in the cell shown in Fig. 10. During the first 60 min after photobleaching, the centrosome recovered ∼50–60% of its original intensity and it then remained at this level for the next six hours.
We could not determine the FRAP characteristics of centrosomes that were photobleached during mitosis. This was because the recovery process was superimposed on natural intensity changes that occurred in the centrosome as the cell progressed through mitosis. Therefore, we conducted this experiment on centrosomes in nocodazole-arrested mitotic cells (n = 7). Under this condition we found that, as during interphase, the centrosome recovered ∼50% of its intensity 30–40 min after photobleaching (Fig. 12 and Fig. 13). However, in contrast to interphase centrosomes, mitotic centrosomes fully recovered to their original intensity within 60–90 min of photobleaching and then remained at that level as long as the cell was blocked in mitosis (Fig. 12 and Fig. 13).
Figure 12.
FRAP experiment in a PtKG cell arrested in mitosis by 4 μM nocodazole. In this cell, one of two separated centrosomes was photobleached (B and C) ∼40 min after nuclear envelope breakdown. The γTGFP signal recovered after photobleaching, indicating that the centrosome-associated γTGFP continued to turn over during mitosis and that this process does not require Mts.
Figure 13.
Centrosome-associated γTGFP intensity versus time in the cell shown in Fig. 12. Note that in contrast to interphase cells, the irradiated centrosome recovered its full intensity over a period of 90 min and then remained at this level.
Discussion
Although the centrosome can be discerned by video-enhanced DIC microscopy in living cells during mitosis, it cannot be distinguished in interphase with certainty from small granules and vacuoles that appear similar in size and contrast. As a result, it is seldom possible to follow the dynamic behavior of this organelle in living cells. With the introduction of GFP-labeling, the position and boundary of the centrosome can now be clearly defined in vivo which, in turn, greatly facilitates studies on centrosome function and behavior (including the isolation of glowing centrosomes). For example, using GFP-labeled γ-tubulin, Ueda et al. 1997 have shown that the centrosome in Dictyostelium does not direct cell migration. Similar methods were also used to demonstrate unique structural changes within the Dictyostelium centrosome as it duplicated and separated during mitosis (Ueda et al. 1999).
To investigate how centrosomes behave during the cell cycle in vertebrates, we have established several cell lines in which this organelle is clearly delineated by γTGFP. As a rule, overexpression of γ-tubulin in vertebrates leads to gross defects and a loss of cell viability (Shu and Joshi 1995). However, modern low-light-level CCD cameras are sensitive enough to detect very few GFP molecules (reviewed in Cubitt et al. 1995; White and Stelzer 1999). By selecting cells expressing only a low-level of the fusion protein, we were able to establish clones that appear normal in every aspect. Thus, substituting at least part of the centrosome's endogenous γ-tubulin with our γTGFP does not deleteriously affect the ability of the centrosome to function normally throughout the cell cycle. The cell cycle-specific redistribution of γTGFP, including its enhanced association with mitotic centrosomes, as well as its transient association with the spindle and midbody, are all consistent with previous immunofluorescence studies of fixed cells (e.g., Stearns et al. 1991; Zheng et al. 1991; Julian et al. 1993; Lajoie-Mazenc et al. 1994).
The Centrosome Contains Two Populations of γ-Tubulin: One Is Stably Bound and the other Is in Dynamic Exchange with a Cytoplasmic Pool
In vertebrates, it has been shown that Mts generated by the centrosome can detach and move away from their site of nucleation (e.g., Vorobjev and Chentsov 1983; Belmont et al. 1990; McBeth and Fujiwara, 1990; Keating et al. 1997). This shedding of Mt minus ends has also been observed in some neuronal cells (Ahmad and Baas 1995) and grasshopper spermatocytes (Ault and Nicklas 1989). Since free Mt minus ends are not stable, it is likely that they are stabilized transiently, perhaps by a γ-TuRC cap. If this is true, then centrosome-associated γ-tubulin must exist in dynamic exchange with a cytoplasmic pool. In this context, only ∼20% of the γ-tubulin within a cell is associated at any one time with the centrosome while the remainder resides in the cytoplasm (Moudjou et al. 1996). In addition, the biochemical properties of centrosomal and cytoplasmic γ-tubulin appear to be similar, and, in both cases, the γ-tubulin exists in large complexes, whose exact composition remains to be determined (Stearns and Kirschner 1994; Zheng et al. 1995; Dictenberg et al. 1998; Oegema et al. 1999).
Using FRAP methodology, we have directly tested the idea that centrosome-associated γ-tubulin is in dynamic exchange. We found that when the γTGFP associated with an interphase centrosome is photobleached, the centrosome recovers ∼50% of it original intensity relatively rapidly (within 60 min), but that the remainder of the photobleached γ-tubulin takes much longer to turn over (greater than five hours). Thus, the centrosome contains two distinct populations of γ-tubulin: one that rapidly exchanges with the cytoplasmic pool and one that is more stable. It is tempting to speculate that the stable population represents γ-tubulin that is allied with the centrioles, while the rapidly exchanging γ-tubulin resides in the pericentriolar material. This is consistent with immunoelectron microscopy data demonstrating γ-tubulin association with the core of centrioles (Fuller et al. 1995; Moudjou et al. 1996) and biochemical studies showing that half of the γ-tubulin is tightly associated with isolated centrosomes, while the other half can be easily extracted (Moudjou et al. 1996).
Our FRAP observations on nocodazole-treated cells reveal that the dynamic exchange of centrosome-associated γ-tubulin occurs even when Mts are not present. This is a surprising finding since, based on prior studies (e.g., Keating et al. 1997), one would have predicted that the rate at which centrosomal γ-tubulin exchanges should depend on Mt dynamics. However, our data clearly reveal that the dynamic exchange of γ-tubulin is not caused by the constant loss of γ-tubulin leaving the centrosome on the tips of released Mts. Instead, centrosomes intrinsically shed γ-tubulin regardless of whether it is associated with the end of a Mt. We also found that the kinetics of exchange do not differ significantly between interphase and mitotic centrosomes, i.e., that the exchangeable population turns over completely within one hour. However, within this time, mitotic centrosomes fully recover their fluorescent intensity, whereas the intensity of interphase centrosomes only recovers to ∼50%. This could mean that mitotic centrosomes no longer possess a nonexchangeable fraction of γ-tubulin. However, an equally plausible explanation is that the nonexchangeable population represents a minor fraction of centrosome-associated γ-tubulin during mitosis. Considering that the γ-tubulin content of the centrosome increases at least threefold at the onset of mitosis, assuming that all of this excess is exchangeable, then the nonexchangeable signal would become diluted to the point that it is no longer detectable by our methods.
Additional γ-Tubulin Is Suddenly Recruited to the Centrosome during Prophase of Mitosis
Early immunofluorescence studies on the distribution of γ-tubulin noted that more of this protein is associated with mitotic than interphase centrosomes (e.g., Zheng et al. 1991). This difference in γ-tubulin content correlates with the fact that mitotic centrosomes generate about five to ten times more Mts than interphase centrosomes (Snyder and McIntosh 1975; Kuriyama and Borisy 1981). When does the centrosome acquire its additional γ-tubulin so that it can generate enhanced numbers of Mts during mitosis? One possibility is that γ-tubulin gradually accumulates in the centrosome during the cell cycle, but it is maintained in an inactive form until spindle formation. The other possibility is that it is suddenly recruited to the centrosome near the onset of mitosis. The former hypothesis has recently been supported by Dictenberg et al. 1998 who concluded, from an immunofluorescence analysis of fixed synchronized CHO cells, that the amount of pericentrin and γ-tubulin associated with the centrosome gradually increases from G1 until mitosis. Our results on living cells are not consistent with this conclusion. Instead, we find that the γ-tubulin content of each centrosome, at best, doubles during interphase, and then suddenly increases more than three times as cells progress through prophase. We also demonstrate that this sudden increase occurs even in the absence of Mts. This means either that the centrosome suddenly acquires the ability to bind more γ-tubulin, or that a sudden global biochemical change within the cell modifies cytoplasmic γ-tubulin so that it binds more efficiently to the centrosomal lattice. Since our FRAP data reveal that recovery occurs with similar kinetics during interphase and mitosis, the affinity of γ-tubulin for the centrosome does not appear to change significantly between these two phases of the cell cycle. Thus, the sudden recruitment of γ-tubulin to the centrosome during prophase is due to changes that occur within the centrosome that allow it to bind more γ-tubulin.
Our results are not inconsistent with the idea that the centrosome, as a structural entity, grows throughout the cell cycle by the gradual accumulation of constituents (e.g., pericentrin). However, our data demonstrate clearly that a key functional component required for enhancing the Mt-nucleating potential of the centrosome during mitosis appears suddenly as the centrosome becomes activated at the G2/M transition. The fact that this process occurs normally in nocodazole-treated cells reveals that the Mt-nucleating potential activity of the centrosome is linked directly to the stage of the cell cycle and not to the functional state of its associated Mt array. This is consistent with accumulating data linking centrosome activation at the G2/M boundary with the phosphorylation of various centrosomal components by CDK1, Polo, and other kinases that regulate progression through the cell cycle (reviewed in Glover et al. 1996; Nigg 1998). In this context, we also note that cells can be induced to return to G2, even after their centrosomes have been activated, as defined by an enhanced accumulation of γ-tubulin. This means that the sudden accumulation of γ-tubulin at the centrosome is not an event that commits the cell to mitosis, i.e., that the cell cycle checkpoint leading to the reversal of prophase can still operate, even after the centrosomes have been activated (see Rieder and Cole 1998a).
While the initial recruitment of γ-tubulin to the centrosome at the G2/M transition is independent of Mts, the presence of Mts leads to subsequent changes in the distribution of γ-tubulin in mitotic cells. We found that, as the spindle formation proceeds, γ-tubulin continues to accumulate around the centrosomes and subsequently spreads into the spindle. This increased γ-tubulin content of the spindle, which has been noted by others on fixed cells (Lajoie-Mazenc et al. 1994), appears in time-lapse recordings to be derived from the centrosome. The accumulation of γ-TGFP in the spindle occurs after the centrosome has reached its maximum fluorescence intensity and is restricted during the initial stages of spindle formation to those parts of half-spindle immediately adjacent to the centrosome (see Fig. 4). Only later, as the spindle becomes compacted during metaphase (see Taylor 1960), does it extend to permeate each half-spindle. This changing pattern of γ-tubulin distribution may arise as each centrosome sheds Mts, capped by γ-tubulin, into its associated half-spindle. Alternatively, the recruitment of γ-tubulin to the spindle may be independent of the centrosome and/or Mt minus ends (Lajoie-Mazenc et al. 1994). Regardless of why the spindle accumulates γ-tubulin, our observations clearly demonstrate that this phenomenon occurs progressively as the spindle matures, and its progress can even be used to distinguish old from young metaphase spindles (adjacent metaphase spindles in Fig. 1).
Our data on living cells also confirm previous reports that γ-tubulin becomes transiently associated with the ends of midbody Mts after cytokinesis. For example, Julian et al. 1993(see also Shu et al. 1995) found that some, but not all, of the midbodies in fixed cell populations labeled with anti–γ-tubulin antibody. They interpreted this to mean that γ-tubulin associates with all midbodies, but only transiently. Our observations, however, suggest that γ-tubulin may become associated with the midbodies in some, but not all, cells and even that this phenomenon may be cell-type specific. Whereas most of the midbodies in our PKG cells contain elevated levels of γ-TGFP, the γ-TGFP content of midbodies in the majority of our CVG and PtKG cells is seldom above background. Importantly, these cells complete cytokinesis normally. Midbody Mts are thought to be derived during anaphase from the centrosomes (Mastronarde et al. 1993). As a result, the accumulation of γ-tubulin at the midbody may be due to the relocation of γ-tubulin, originally associated with spindle Mts, as these Mts become concentrated in the midzone during cytokinesis. Under this scenario, the presence or absence of γ-tubulin in the midbody may simply manifest how rapidly this molecule dissociates from the midzone Mts.
Acknowledgments
We thank Dr. Berl Oakley (Ohio State University) for providing us with the human γ-tubulin clone, Mr. Richard Cole for help in assembling the FRAP apparatus, and Drs. Michael Koonce, Bruce McEwen, and Sam Bowser for critical editorial comments on the manuscript. We also gratefully acknowledge use of the Wadsworth Center's Video LM and Molecular Genetics core facilities.
The work reported here was supported, in part, by the National Institutes of Health grants GMS R01 59363 to A. Khodjakov and GMS R01 40198 to C.L. Rieder.
Footnotes
1.used in this paper: γTGFP, γ-tubulin/green fluorescence protein; γTuRC, γ-tubulin ring complex; FRAP, fluorescence recovery after photobleaching; GFP, green fluorescent protein; LM, light microscopy; Mt, microtubule
References
- Ahmad F.J., Baas P.W. Microtubules released from the neuronal centrosome are transported into the axon. J. Cell Sci. 1995;108:2761–2769. doi: 10.1242/jcs.108.8.2761. [DOI] [PubMed] [Google Scholar]
- Ault J.G., Nicklas R.B. Tension, microtubule rearrangements, and the proper distribution of chromosomes in mitosis. Chromosoma. 1989;98:33–39. doi: 10.1007/BF00293332. [DOI] [PubMed] [Google Scholar]
- Belmont L.D., Hyman A.A., Sawin K.E., Mitchison T.J. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990;62:579–589. doi: 10.1016/0092-8674(90)90022-7. [DOI] [PubMed] [Google Scholar]
- Brinkley B.R., Cox S.M., Pepper D.A., Wible L., Brenner S.L., Pardue R.L. Tubulin assembly sites and the organization of cytoplasmic microtubules in cultured mammalian cells. J. Cell Biol. 1981;90:554–562. doi: 10.1083/jcb.90.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze V.E., Borisy G.G. Nucleation of microtubules from mitotic centrosomes is modulated by a phosphorylated epitope. J. Cell Sci. 1990;95:405–411. doi: 10.1242/jcs.95.3.405. [DOI] [PubMed] [Google Scholar]
- Cubitt A.B., Heim R., Adams S.R., Boyd A.E., Gross L.A., Tsien R.Y. Understanding, improving and using green fluorescent protein. Trends Biochem. 1995;20:448–455. doi: 10.1016/s0968-0004(00)89099-4. [DOI] [PubMed] [Google Scholar]
- Dictenberg J.B., Zimmerman W., Sparks C.A., Young A., Vidair C., Zheng Y., Carrington W., Fay F.S., Doxsey S.J. Pericentrin and γ-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J. Cell Biol. 1998;141:163–174. doi: 10.1083/jcb.141.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey S.J., Stein P., Evans L., Calarco P.D., Kirschner M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 1994;76:639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- Felix M.A., Antony C., Wright M., Maro B. Centrosome assembly in vitrorole of γ-tubulin recruitment in Xenopus sperm aster formation. J. Cell Biol. 1994;124:19–31. doi: 10.1083/jcb.124.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S.D., Gowen B.E., Reinsch S., Sawyer A., Buendia B., Wepf R., Karsenti E. The core of the mammalian centriole contains gamma-tubulin. Curr. Biol. 1995;5:1384–1393. doi: 10.1016/s0960-9822(95)00276-4. [DOI] [PubMed] [Google Scholar]
- Gard D.L., Hafezi S., Zhang T., Doxsey S.J. Centrosome duplication continues in cycloheximide-treated Xenopus blastulae in the absence of a detectable cell cycle. J. Cell Biol. 1990;110:2033–2042. doi: 10.1083/jcb.110.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover D.M., Ohkura H., Tavares A. Polo kinasethe choreographer of the mitotic stage? J. Cell Biol. 1996;135:1681–1684. doi: 10.1083/jcb.135.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R.R., Borisy G.G. The pericentriolar material in Chinese hamster ovary cells nucleates microtubule formation. J. Cell Biol. 1977;73:601–615. doi: 10.1083/jcb.73.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchcliffe E.H., Li C., Thompson E.A., Maller J.L., Sluder G. Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extract. Science. 1999;283:851–854. doi: 10.1126/science.283.5403.851. [DOI] [PubMed] [Google Scholar]
- Julian M., Tollon Y., Lajoie-Mazenc I., Moisand A., Mazarguil H., Puget A., Wright M. γ-Tubulin participates in the formation of the midbody during cytokinesis in mammalian cells. J. Cell Sci. 1993;105:145–156. doi: 10.1242/jcs.105.1.145. [DOI] [PubMed] [Google Scholar]
- Keating T.J., Peloquin J.G., Rodionov V.I., Momcilovic D., Borisy G.G. Microtubule release from the centrosome. Proc. Natl. Acad. Sci. USA. 1997;94:5078–5083. doi: 10.1073/pnas.94.10.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D.R., Moritz M., Alberts B.M. The centrosome and cellular organization. Ann. Rev. Biochem. 1994;63:639–674. doi: 10.1146/annurev.bi.63.070194.003231. [DOI] [PubMed] [Google Scholar]
- Khodjakov A., Cole R.W., Rieder C.L. A synergy of technologiescombining laser microsurgery with green fluorescent protein tagging. Cell Motil. Cytoskelet. 1997;38:311–317. doi: 10.1002/(SICI)1097-0169(1997)38:4<311::AID-CM1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kuriyama R., Borisy G.G. Microtubule-nucleating activity of centrosomes in Chinese hamster ovary cells is independent of the centriole cycle but coupled to the mitotic cycle. J. Cell Biol. 1981;91:822–826. doi: 10.1083/jcb.91.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey K.R., Jackson P.K., Stearns T. Cyclin-dependent kinase control of centrosome replication. Proc. Natl. Acad. Sci. USA. 1999;96:2817–2822. doi: 10.1073/pnas.96.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie-Mazenc I., Tollon Y., Detraves C., Julian M., Moisand A., Gueth-Hallonet C., Debec A., Salles-Passador I., Puget A., Mazarguil H. Recruitment of antigenic γ-tubulin during mitosis in animal cellspresence of γ-tubulin in the mitotic spindle. J. Cell Sci. 1994;107:2825–2837. doi: 10.1242/jcs.107.10.2825. [DOI] [PubMed] [Google Scholar]
- Manders E.M.M., Kimura H., Cook P.R. Direct imaging of DNA in living cells reveals the dynamics of chromosome formation. J. Cell Biol. 1999;144:813–822. doi: 10.1083/jcb.144.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde D.N., McDonald K.L., Ding R., McIntosh J.R. Interpolar spindle microtubules in PTK cells. J. Cell Biol. 1993;123:1475–1489. doi: 10.1083/jcb.123.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D. The chromosome cycle and the centrosome cycle in the mitotic cycle. Int. Rev. Cytol. 1987;100:49–92. doi: 10.1016/s0074-7696(08)61698-8. [DOI] [PubMed] [Google Scholar]
- McBeath E., Fujiwara K. Microtubule detachment from the microtubule-organizing center as a key event in the complete turnover of microtubules in cells. Eur. J. Cell Biol. 1990;52:1–16. [PubMed] [Google Scholar]
- Moritz M., Braunfeld M.B., Sedat J.W., Alberts B., Agard D.A. Microtubule nucleation by gamma-tubulin-containing rings in the centrosome. Nature. 1995;378:638–640. doi: 10.1038/378638a0. [DOI] [PubMed] [Google Scholar]
- Moritz M., Zheng Y., Alberts B.M., Oegema K. Recruitment of the γ-tubulin ring complex to Drosophila salt-stripped centrosome scaffolds. J. Cell Biol. 1998;142:775–786. doi: 10.1083/jcb.142.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudjou M., Bordes N., Paintrand M., Bornens M. γ-Tubulin in mammalian cellsthe centrosomal and the cytosolic forms. J. Cell Sci. 1996;109:875–887. doi: 10.1242/jcs.109.4.875. [DOI] [PubMed] [Google Scholar]
- Nigg E.A. Polo-like kinasespositive regulators of cell division from start to finish. Curr. Opin. Cell Biol. 1998;10:776–783. doi: 10.1016/s0955-0674(98)80121-x. [DOI] [PubMed] [Google Scholar]
- Oakley C.E., Oakley B.R. Identification of gamma-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans . Nature. 1989;338:662–664. doi: 10.1038/338662a0. [DOI] [PubMed] [Google Scholar]
- Oegema K., Wiese C., Martin O.C., Iwamatsu A., Mitchison T.J., Zheng Y. Characterization of two related Drosophila γ-tubulin complexes that differ in their ability to nucleate microtubules. J. Cell Biol. 1999;144:721–733. doi: 10.1083/jcb.144.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti A., Bornens M. Organisation and functional regulation of the centrosome in animal cells. Prog. Cell Cycle Res. 1997;3:285–299. doi: 10.1007/978-1-4615-5371-7_23. [DOI] [PubMed] [Google Scholar]
- Rieder C.L., Borisy G.G. The centrosome cycle in PtK2 cellsasymmetric distribution and structural changes in the pericentriolar material. Biol. Cell. 1982;44:117–132. [Google Scholar]
- Rieder C.L., Cole R.W. Entry into mitosis in vertebrate somatic cells is guarded by a chromosome damage checkpoint that reverses the cell cycle when triggered during early, but not late, prophase J. Cell Biol. 142 1998. 1013 1022a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C.L., Cole R.W. Perfusion chambers for high-resolution video-light microscopic studies of vertebrate cell monolayerssome considerations and a design Sluder G., Wolf D.E. Video Microscopy 1998. 253 275 Academic Press Inc; New York: b [DOI] [PubMed] [Google Scholar]
- Rieder C.L., Schultz A., Cole R., Sluder G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J. Cell Biol. 1994;127:1301–1310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C.L., Cole R.W., Khodjakov A., Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnackenberg B.J., Khodjakov A., Rieder C.L., Palazzo R.E. The disassembly and reassembly of functional centrosomes in vitro . Proc. Natl. Acad. Sci. USA. 1998;95:9295–9300. doi: 10.1073/pnas.95.16.9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H.B., Joshi H.C. γ-Tubulin can both nucleate microtubule assembly and self-assemble into novel tubular structures in mammalian cells. J. Cell Biol. 1995;130:1137–1147. doi: 10.1083/jcb.130.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H.B., Li Z., Palacios M.J., Li Q., Joshi H.C. A transient association of gamma-tubulin at the midbody is required for the completion of cytokinesis during the mammalian cell division. J. Cell Sci. 1995;108:2955–2962. doi: 10.1242/jcs.108.9.2955. [DOI] [PubMed] [Google Scholar]
- Sluder G., Rieder C.L. Controls for centrosome reproduction in animal cellsissues and recent observations. Cell Motil. Cytoskelet. 1996;33:1–5. doi: 10.1002/(SICI)1097-0169(1996)33:1<1::AID-CM1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Snyder J.A., McIntosh J.R. Initiation and growth of microtubules from mitotic centers in lysed mammalian cells. J. Cell Biol. 1975;67:744–760. doi: 10.1083/jcb.67.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T., Kirschner M. In vitro reconstitution of centrosome assembly and functionthe central role of gamma-tubulin. Cell. 1994;76:623–637. doi: 10.1016/0092-8674(94)90503-7. [DOI] [PubMed] [Google Scholar]
- Stearns T., Evans L., Kirschner M. γ-tubulin is a highly conserved component of the centrosome. Cell. 1991;65:825–836. doi: 10.1016/0092-8674(91)90390-k. [DOI] [PubMed] [Google Scholar]
- Taylor E.W. Dynamics of spindle formation. Ann. NY. Acad. Sci. 1960;90:430–434. doi: 10.1111/j.1749-6632.1960.tb23261.x. [DOI] [PubMed] [Google Scholar]
- Ueda M., Graf R., MacWilliams H.K., Schliwa M., Euteneuer U. Centrosome positioning and directionality of cell movements. Proc. Natl. Acad. Sci. USA. 1997;94:9674–9678. doi: 10.1073/pnas.94.18.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M., Schliwa M., Euteneuer U. Unusual centrosome cycle in Dictyosteliumcorrelation of dynamic behavior and structural changes. Mol. Biol. Cell. 1999;10:151–160. doi: 10.1091/mbc.10.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjev I.A., Chentsov Y.S. The dynamics of reconstitution of microtubules around the cell center after cooling. Eur. J. Cell Biol. 1983;30:149–153. [PubMed] [Google Scholar]
- White J., Stelzer E. Photobleaching GFP reveals protein dynamics inside live cells. Trends Cell Biol. 1999;9:61–65. doi: 10.1016/s0962-8924(98)01433-0. [DOI] [PubMed] [Google Scholar]
- Zhai Y., Borisy G.G. Quantitative determination of the proportion of microtubule polymer present during the mitosis-interphase transition. J. Cell Sci. 1994;107:881–890. doi: 10.1242/jcs.107.4.881. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Jung M.K., Oakley B.R. γ-tubulin is present in Drosophila melanogaster and Homo sapiens and is associated with the centrosome. Cell. 1991;65:817–823. doi: 10.1016/0092-8674(91)90389-g. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Wong M.L., Alberts B., Mitchison T. Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]